Abstract
Summary: Marchiafava-Bignami disease (MBD), a rare complication of chronic alcoholism, is characterized by primary demyelination of the corpus callosum. We report two cases of MBD in which fluid-attenuated inversion recovery (FLAIR) and diffusion-weighted imaging studies revealed symmetrical hyperintense lesions in the cerebral cortex (particularly in the lateral-frontal regions) in addition to the callosal lesions, which suggests an association of diffuse cortical lesions such as Morel’s laminar sclerosis with MBD.
Marchiafava-Bignami disease (MBD), which results in demyelination of the corpus callosum, can be observed in people with chronic alcoholism. Although most previous reports of MBD have been based on postmortem pathologic findings (1), the advent of MR imaging has allowed detailed analysis of the distribution of lesions (2, 3). Recent MR imaging studies have shown that lesions may also be found in the hemispheric white matter (3, 4). To the best of our knowledge, however, there has been no reported patient with MBD in whom MR imaging showed cortical abnormalities in addition to callosal lesions. We describe the MR imaging findings in the cerebral cortex of two patients with MBD.
Case Reports
Patient 1
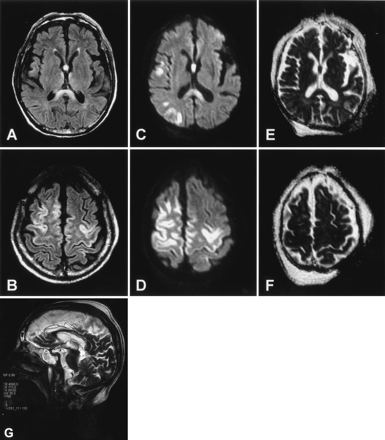
A 72-year-old man with a 40-year history of alcohol (Japanese sake) abuse experienced confusion, lethargy, and diminished appetite for approximately 1 week. He was hospitalized and referred to our department because of his rapidly decreasing level of consciousness. On examination, he was emaciated, dehydrated, and in a deep coma. Results of cerebral spinal fluid (CSF) studies were normal, and electroencephalography (EEG) showed generalized ς rhythm intermingled with δ waves. Fluid-attenuated inversion recovery (FLAIR) and diffusion-weighted images obtained on admission showed hyperintense lesions involving the splenium and body of the corpus callosum (Fig 1). In addition, symmetrical cerebral cortical hyperintense lesions were observed mainly in lateral-frontal regions on FLAIR and diffusion-weighted images (Fig 1). Apparent diffusion coefficient (ADC) mapping showed relatively hypointensity in the callosal and cortical lesions (Fig 1E, -F). On the basis of clinical history and imaging features, MBD was diagnosed, and high-dose vitamin B complex including 500 mg/day thiamine was administered intravenously for 7 days. The patient, however, remained in a persistent vegetative state for more than 3 months. Follow-up T2-weighted sagittal MR imaging study 10 days after the initial study confirmed that lesions were most extensive in the central parts of the corpus callosum, with relative sparing of the dorsal and ventral layers (Fig 1G).
MR images in patient 1. Axial FLAIR images on admission show hyperintensity in the corpus callosum (A) and the cerebral cortex (B). Diffusion-weighted images (C and D) also show hyperintensity in these regions with relatively decreased ADC values (see Fig 3). The decreased ADC values, however, are inconspicuous on ADC mapping (E and F). Follow-up T2-weighted sagittal image obtained 10 days after the initial study shows callosal lesions mainly involving the central part of the splenium and body (G).
Patient 2
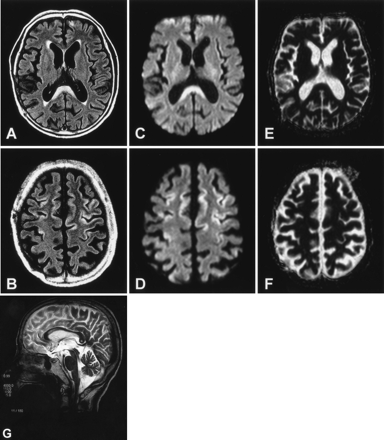
A 56-year-old man was admitted to our hospital because of acute onset of seizures and altered mental state. He had abused alcohol (Japanese sake) for 30 years. At examination, the patient was confused. Although there was no weakness in his extremities, he showed gait disturbance and lack of motor coordination. Routine blood tests and CSF studies revealed no abnormalities. EEG showed diffuse slow waves of 6–8 Hz without epileptiform discharge. FLAIR, and diffusion-weighted images showed abnormal hyperintensity in the corpus callosum and lateral-frontal cerebral cortices with relatively reduced ADC, findings similar to those in case 1 (Fig 2).
MR images in patient 2. Axial FLAIR images on admission show hyperintensity in the corpus callosum (A) and the cerebral cortex (B). Diffusion-weighted images (C and D) also show hyperintensity in these regions with relatively decreased ADC values (see Fig 3). The decreased ADC values, however, are inconspicuous on ADC mapping (E and F). Follow-up T2-weighted sagittal image obtained 10 days after the initial study shows callosal lesions mainly involving the central part of the splenium and body (G).
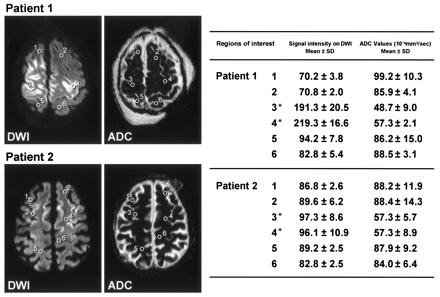
Comparison of signal intensities on diffusion-weighted images with ADC values on ADC maps. Each coordinate of regions of interest in the ADC maps is the same as that in diffusion-weighted images. ADC values are reduced in regions with high signal intensity on diffusion-weighted images (regions 3 and 4 in each patient). Asterisks denote regions in affected cortex identified by high signal intensity on diffusion-weighted images.
Although the patient was given high-dose vitamin B complex, including 500 mg/day thiamine, intravenously for 7 days with 3 days of high-dose intravenous corticosteroids, he remained in an apathetic state for more than 3 months with long- and short-term memory deficits.
Discussion
The main pathologic findings in MBD consist of symmetrical demyelination and necrosis of the central part of the corpus callosum, with relative sparing of thin upper and lower edges (5). The diagnosis of MBD rests mainly on evidence of these callosal lesions. The corpus callosum may also be affected in other diseases such as ischemic stroke, contusion, multiple sclerosis, and lymphoma. MBD, however, is distinguished from these disorders by the symmetry of the callosal lesions (6). MR imaging is the best technique with which to evaluate such MBD lesions, and it showed these neuroradiologic features characteristic of MBD in both our patients.
A highly unusual feature in our patients was the diffuse cortical abnormality of the lateral-frontal cerebral cortices. In a few cases of Wernicke’s encephalopathy, another alcoholism-induced encephalopathy, characterized by lesions involving medial thalami, mamillary bodies, and periaqueductal brain stem, cortical abnormality has been described (7). Our patients’ MR images did not show the midline lesions characteristic of Wernicke’s encephalopathy. The coexistence of subclinical or an extremely mild form of Wernicke’s encephalopathy cannot be ruled out in our patients. With Wernicke’s encephalopathy, the cortical lesions are usually restricted to the motor and premotor cortices (7). Thus, our patients’ diffuse cortical lesions are more likely associated with MBD than with Wernicke’s encephalopathy.
Postmortem neuropathologic study in patients with MBD sometimes reveals a type of cerebral cortical lesion (8, 9). This cortical lesion, known as Morel’s laminar sclerosis, is characterized by cortical laminar necrosis and gliosis, mainly in the third layer and especially in the lateral-frontal cortex (10). Although Morel’s laminar sclerosis was reported as the only manifestation of alcoholic encephalopathy in one patient (11), it is usually associated with, and probably secondary to, the callosal lesions of MBD (10).
In contrast to postmortem neuropathologic study findings, cortical abnormality in MBD is rarely reported on the basis of intravital neuroradiologic examination. There is one report of a severe positron-emission tomography–detected decrease in cortical metabolism in a patient with MBD (12). Cortical abnormality detected by MR imaging, however, has not been reported previously in patients with MBD. Morel’s laminar sclerosis is seen mainly in the lateral-frontal cortices (10). Our patients’ MR imaging abnormalities were also seen mainly in the lateral-frontal cortices and thus may reflect Morel’s laminar sclerosis. If this assumption is correct, a high signal intensity on diffusion-weighted images with relatively reduced ADC in the cortical lesions (Fig 3) suggests the pathologic change of the acute phase of Morel’s laminar sclerosis to be a cytotoxic edema; however, further studies are necessary to delineate the pathologic details of cortical lesions associated with MBD.
References
- Received April 16, 2004.
- Accepted after revision June 18, 2004.
- Copyright © American Society of Neuroradiology