Abstract
Summary: Left atrial myxoma commonly leads to cerebral embolic ischemic stroke. The subsequent formation of cerebral aneurysms due to myxomatous emboli is a phenomenon that may lead to severe neurologic complications such as intracerebral hemorrhage. In addition to the formation of aneurysms in cerebral arteries, we report here the unique picture of a retinal involvement consisting in microaneurysm formation associated with myxomatous embolism.
Atrial myxomas represent approximately 50% of all cardiac tumors, occurring mainly in the 3rd–6th decade of life (1). They originate from subendocardial mesenchymal cells mainly from the left atrium. Patients with left atrial myxoma usually present with signs of cardiac failure due to obstructed ventricular filling causing dyspnea, pulmonary edema, and right heart failure. In some cases, it leads to syncope, sudden death, or signs of systemic embolism.
The embolization of tumor particles or thrombotic material covered with tumor cells occurs in 30–45% of myxoma patients. In at least half of the cases cerebral arteries are affected, leading to embolic ischemic stroke (2). In contrast, the formation of intracranial aneurysms associated with left atrial myxomas is a less common phenomenon. Other rare neurologic complications include parenchymal brain metastases and intracerebral hemorrhage due to ruptured aneurysms (3, 4). We report the case of a young male patient who presented with cerebral embolic ischemic stroke and showed the angiographic picture of multiple cerebral aneurysms. In addition, microaneurysms of retinal arterioles were demonstrated by fluorescein angiography.
Case Report
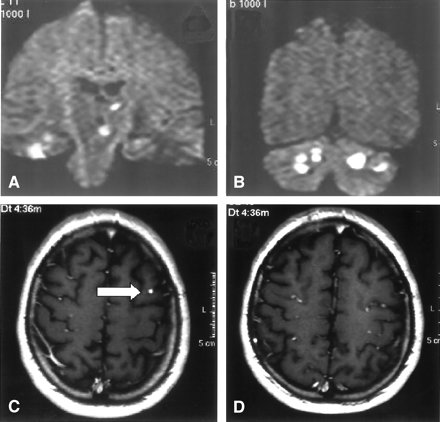
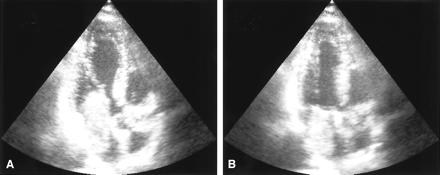
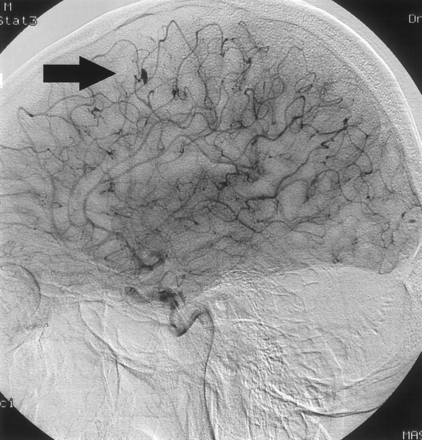
A 31-year-old man was admitted to our hospital with acute onset of dizziness, nausea, blurred vision of his left eye, and gait disturbance. On physical examination, he showed postural imbalance with tendency to fall to the right, dysarthria, skew deviation with a lower left bulb, and mild left-sided facial weakness. On cardiac examination, he had a regular rate and rhythm. No murmurs, rubs, or gallops were audible on auscultation. Duplex sonography gave normal results for his carotid, basilar, and vertebral arteries. Electrocardiography demonstrated signs of left atrial enlargement; a chest radiograph was normal. Holter electrocardiogram showed few ventricular and supraventricular extrasystoles but was otherwise regular. CSF results were normal, with no signs of hemorrhage or cytological abnormalities. Erythrocyte sedimentation rate was 30 mm after 1 hour. A preoperatively performed Coombs test was positive, with detection of cold agglutinins (anti-P1 antibody). All other laboratory results were normal. Cerebral MR imaging revealed multiple cerebellar and brain stem infarctions (Fig 1A, -B) as well as some smaller bihemispheric subcortical white matter lesions on T2-weighted images (not shown). There were no signs of intracranial bleeding. Gadolinium-enhanced T1-weighted sequences showed multiple small enhancing lesions probably corresponding to dilated blood vessels (Fig 1C, -D). Subsequent transthoracic and transesophageal echocardiography demonstrated a large mass with a suspected size of 2.5 × 2.5 × 7 cm in the left atrium, which prolapsed through the mitral valve into the left ventricle, suggesting the diagnosis of atrial myxoma (Fig 2). Cerebral angiography showed multiple intracranial microaneurysms up to 6 mm in diameter mainly localized in peripheral branches of middle, anterior, and posterior cerebral arteries; a few aneurysms were seen in branches of the vertebrobasilar arteries (Fig 3). One week after the initial neurologic event, fundoscopy was normal. A retinal fluorescein angiography revealed parafoveal teleangiectasias and microaneurysms in both eyes (Fig 4). Open heart surgery was performed, entirely removing the gelatinous tumor. Histologic examination confirmed the diagnosis of myxoma. After surgery, the patient was discharged from hospital without any specific treatment and recovered completely. Cerebral MR imaging 2 weeks after the operation showed the residues of cerebral infarctions and the cerebral aneurysm unchanged compared with the prior MR images. No new cerebral ischemic lesions were detected. Two years after the initial event, he was evaluated in the neurologic outpatient clinic and classified as being well, without any residual or newly developed symptoms.
MR images showing infarction and dilated blood vessels. A and B, Coronal diffusion-weighted MR imaging (TR, 5000 ms; TE, 120 ms; Td, 85 ms; section thickness, 5 mm) shows multiple cerebellar and brain stem infarcts. C and D, Axial T1-weighted spin-echo MR imaging following gadolinium-DTPA administration (TR, 560 ms; TE, 14 ms; section thickness, 5 mm; interslice gap, 1 mm) demonstrates dilated peripheral middle cerebral artery branches suggestive of fusiform dilatations (arrow).
Echocardiography demonstrating left atrial myxoma. Transesophageal echocardiogram in the two chamber view in diastole (A) and systole (B). A large intraatrial mass which prolapses through the mitral valve into the left ventricle in diastole (A) can be seen.
Digital subtraction angiography confirming aneurysm formation. The largest aneurysm is located in the precentral branch of the left middle cerebral artery (arrow).
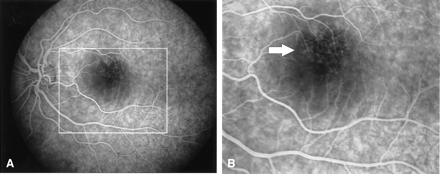
Fluorescein angiography of the left eye with parafoveal teleangiectasis or microaneurysms. A and B, Early-phase fluorescein angiogram demonstrates teleangiectasis of the upper perifoveal capillary network (arrow). Similar changes were observed in the right eye. A detail from panel A, marked by a rectangle, is shown in panel B. Visual acuity was 25/25.
Discussion
Atrial myxomas have been estimated to cause up to 0.5% of ischemic strokes (5). In a recently published series, the median delay between onset of symptoms and diagnosis in myxoma patients with neurologic manifestation—mainly transient ischemic attacks—was 36 months (6). Cerebral imaging often demonstrates multiple infarcts suggestive of an embolic cause, but in some cases it may show only small subcortical ischemic lesions mimicking lacunar disease (7). Transthoracic echocardiography has a sensitivity of around 90% in detection of left atrial myxoma; the sensitivity of transesophageal examination is even higher (8).
To the best of our knowledge, fewer than 20 cases with intracranial aneurysms associated with myxoma have been reported in the literature (4, 9–11). Several possible mechanisms of aneurysm formation have been proposed. Tumor cells may infiltrate cerebral vessels via vasa vasorum, thereby destroying the architecture of arterial walls similar to the mechanism of mycotic aneurysms. Vasa vasorum, however, are scarcely found in intracranial arteries of experimental animals or humans (12). Alternatively, perivascular damage may be due to vascular occlusion by tumor material with subsequent scarring and pseudoaneurysm formation. This explanation is, however, challenged by the observation that most reported aneurysms occurred without a previous history of cerebrovascular embolization. Another proposed mechanism is the direct transendothelial invasion of tumor cells into the arterial wall causing destruction of its architecture and subsequent aneurysm dilatation. Some histopathologic studies indeed detected myxoma cells in the wall of aneurysms, with one study directly demonstrating interruption of the internal elastic lamina by invading tumor cells (13). In our patient, aneurysms formed without previous symptoms of cerebral embolism. This supports the hypothesis that aneurysm formation and vascular occlusion are not necessarily associated but occur as independent complications of left atrial myxoma; however, clinically silent cerebrovascular occlusion and subsequent ischemic damage cannot be totally excluded.
A number of cases of myxomatous embolism to ocular arteries with a predilection for the left eye and in some cases with retinal hemorrhage have been reported (14). It is conceivable that the retinal vascular dilatations observed in our patient indeed represent microaneurysms corresponding to the intracranial aneurysms (Fig 3). To the best of our knowledge, this is the first report demonstrating retinal microaneurysm formation associated with atrial myxoma.
Conclusion
Because reports covering long-term follow-up of patients with cerebral myxomatous aneurysms associated are rare, recommendations for postoperative management for this subgroup are problematic. Although published case series show that immediate central nervous manifestations are often severe and may be fatal (15), they also indicate a good long-term prognosis with respect to recurrence of neurologic events (2, 5, 6, 15, 16); however, several cases of rapid growth of preexisting aneurysms or postoperative formation of new aneurysms, as well as recurrent cerebral hemorrhages up to years after atrial myxoma resection, have been reported (3, 13, 17, 18). Spontaneous thrombosis resulting in resolution of an aneurysm has been reported once (19). Altogether, these reports indicate that postoperative neurologic complications may occur even years after myxoma resection but are probably rare. Careful follow-up of patients with known aneurysms is recommended, and large aneurysms may require invasive treatment. In view of the persistent risk of embolism, aneurysm formation, and other systemic complications, on one hand, and the good postoperative prognosis, on the other hand, early diagnosis of cardiac myxoma is desirable (1, 16).
References
- Received April 15, 2004.
- Accepted after revision June 23, 2004.
- Copyright © American Society of Neuroradiology