Abstract
BACKGROUND AND PURPOSE: The recent advent of flexible stents has enabled their application in intracranial atherosclerotic disease. However, it is unclear whether perforating artery occlusion occurs after stent placement in atherosclerotic stenotic vessels. We investigated this issue by using experimental atherosclerosis-induced rabbits.
METHODS: A stainless steel balloon-expandable stent was deployed into the atherosclerosis-induced abdominal aorta across the lumbar artery in six New Zealand white rabbits. This model system is suitable because the diameter of the abdominal aorta is similar to that of human intracranial arteries. We evaluated the patency of the lumbar artery by using angiography and scanning electron microscopy (SEM) 3 months after stent placement. Histopathologic evaluation also was performed in one rabbit.
RESULTS: The lumbar artery was patent in five of six rabbits per angiography. The lumbar artery was occluded with an intraluminal thrombus in one rabbit. However, SEM findings demonstrated that the stent struts were covered completely with a thick neointima and the ostium of the lumbar artery became narrowed in all cases. In the one lumbar artery that was occluded at angiography, histopathologic findings confirmed that intraluminal thrombus surrounded the stent struts crossing the ostium.
CONCLUSION: We observed luminal narrowing after stent placement in an atherosclerotic stenotic vessel, although patency of the perforating arteries was generally maintained.
Intracranial atherosclerotic diseases are known to cause future strokes with a high rate, despite anticoagulation or antiplatelet therapy (1–3). Moreover, an international randomized trial proved that surgical bypass surgery was not effective for the prevention of future strokes (4). However, percutaneous transluminal angioplasty, which can recover anterograde blood flow, is a candidate as a treatment for intracranial atherosclerotic disease. In addition, the development of flexible stents has permitted the endovascular treatment of intracranial atherosclerotic diseases, and many successful cases have been reported (5–14). Stent placement for intracranial atherosclerotic vessels, however, is not yet widely used, owing in part to a number of unresolved problems. One major issue is whether perforating arteries remain patent after stent placement. Using a normal rabbit model, we previously reported that stent placement does not compromise the patency of the perforating artery if the stent struts do not completely cover the ostium of the perforating arteries (15). However, the neointimal response after stent placement in atherosclerotic vessels is quite different from the response of normal vessels (16). Thus, we investigated whether the patency of the perforating arteries will be maintained after stent placement in atherosclerotic vessels.
Methods
We previously reported that the spatial relationship between the abdominal aorta and the lumbar arteries in rabbit is anatomically similar to that of the major intracranial arteries and perforating arteries in humans, with regard to the diameter and branching angle (15). Thus, we placed stents across the ostium of the lumbar artery in the atherosclerosis-induced abdominal aorta in rabbits and evaluated the patency of the lumbar arteries by using angiography and scanning electron microscopy (SEM), as well as histopathologic methods. All animal care conformed to the institutional guidelines of Wakayama Medical University, Wakayama, Japan.
Induction of Atherosclerosis
To induce atherosclerotic changes in rabbit aorta, we used previously described methods with modifications (17–19). Six New Zealand white rabbits weighing 2–3 kg were initially fed a high-cholesterol diet (2% cholesterol, 3% coconut oil) for 4 weeks. Afterward, the rabbits underwent balloon denudation of their abdominal aorta. For this procedure, the rabbits were anesthetized by means of an intravenous injection of pentobarbital 4 mg/kg. The right femoral artery was exposed, and a 4F sheath was inserted through a femoral arteriotomy. After angiography was performed as a control, the endothelium of the abdominal aorta was denuded by withdrawing an inflated 3F Fogarty balloon catheter (Baxter, Irvine, CA) three times. Postprocedural angiography was performed, and the surgical procedure was finished. Before balloon denudation, heparin 100 U/kg was given intravenously. Aspirin 5 mg/kg was administered orally from 1 day before the surgery to 1 month after stent placement. All rabbits were continuously fed a high-cholesterol diet for 6 weeks after balloon denudation.
Stent Placement
Stents were implanted 6 weeks after balloon denudation. The atherosclerotic rabbits underwent follow-up angiography through the left femoral artery. First, the stenosis ratio was determined by using a measure wire placed in the aorta. The stenosis ratio was defined as follows: 1 minus the diameter of the vessel before stent placement divided by the diameter of the vessel before denudation. The stainless steel balloon expandable stent (3.0 x 15 mm, Multi-Link; Guidant/Advanced Cardiovascular Systems Inc., Temecula, CA) was deployed through a femoral artery by using a 0.014-inch guidewire (Transend; Boston Scientific, Natick, MA) at the most severely stenotic portion of the abdominal aorta that involved the lumbar artery. The characteristics of this stent include good conformability due to the very thin flat strut. The rabbits were systemically heparinized (100 U/Kg) before stent placement. The stent was deployed at 6–8 atm for 15 seconds. Postprocedural angiography was performed, and the surgery was completed.
Follow-up Angiography
Follow-up angiography was performed through the left common carotid artery 3 months after stent placement to evaluate the lumbar artery patency.
Scanning Electron Microscopy
After follow-up angiography, five of the six rabbits were euthanized for SEM analysis, as previously described. Inferior vena cava exsanguination was performed by perfusion with 0.2 mol/L phosphate buffered saline (PBS) with heparin through the left ventricular puncture. The arteries with stents then were harvested and fixed with 0.1 mol/L PBS, containing 1% paraformaldehyde and 1.25% glutaraldehyde. After rinsing with 0.1 mol/L PBS, the arteries with stents were gently opened with tungsten scissors to prevent intraluminal damage, and the specimens were postfixed with 1% OsO4 at 4°C for 1 hour. The arteries with stents were then dehydrated with graded ethanol and t-butyl alcohol, and then freeze-dried. The specimens were coated with 20 nm of gold, and the patency of the lumbar artery and the neointimal thickness were examined.
Histopathologic Evaluation
The aorta with stent in one rabbit was examined at the level of the lumbar artery ostium by using histopathologic methods. After exsanguination, the artery with stent was harvested and fixed with 10% neutral buffered formalin. This artery was carefully sectioned with a tungsten knife at 4–5 μm thickness. The sections were stained with hematoxylin-eosin and Masson-Trichrome to examine the cellular composition.
Results
Induced Aortic Atherosclerosis
The vessel wall of the denuded aorta increased in thickness macroscopically 6 weeks after balloon denudation. Microscopic findings revealed neointimal hyperplasia with disruption of the internal elastic lamina. The neointima consisted mainly of hypertrophic smooth muscle cells and connective tissue (data not shown). The denuded aorta demonstrated atherosclerotic changes with segmental stenotic lesions at angiography.
Angiographic Findings of the Aorta with Stent
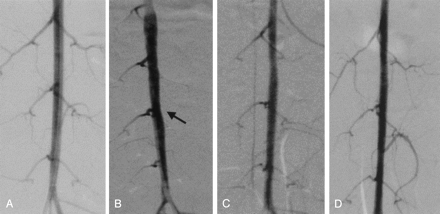
The angiographic results are summarized in the Table. The average stenosis ratio of the most severely affected abdominal aorta involving the lumbar artery was 25%. In all cases, the lumbar arteries were patent without late filling before and just after stent placement per angiography. Follow-up angiography demonstrated that the lumbar arteries in five of six rabbits were still patent without late filling. However, the lumbar artery in one rabbit was completely occluded. Except for this one case, restenosis of the aorta with stent was not encountered at follow-up angiography. The angiographic changes of a representative case are shown in Figure 1.
Angiographic change in a representative case (case 1).
A–D, Angiograms obtained after denudation (A), 6 weeks after denudation but before stent placement (B), immediately after stent placement (C), and at follow-up 3 months after stent placement (D). Arrow in B indicates stenotic lesion before stent placement.
Summary of experimental cases
SEM Findings
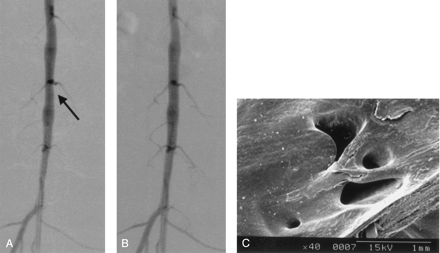
SEM was performed in five rabbits to investigate the pathomorphologic status at the lumbar artery ostium. The stent strut crossed the lumbar artery ostium in all cases. Although the ostium of the lumbar artery remained patent, luminal narrowing was shown in all cases and the stent strut was completely covered with a thick neointima. In addition, the small ostial space, bridged by the stent strut, became stenotic due to the neointimal proliferation. The angiographic and SEM findings of the same rabbit are shown in Figure 2, and the SEM findings in another rabbit are shown in Figure 3.
Angiographic change and SEM findings in a representative case (case 2).
A, Prestenting angiogram shows stenotic change at the level of the lumbar artery orifice (arrow).
B, Angiogram obtained after stent placement shows that the stenotic lesion is improved and the lumbar artery is patent.
C, SEM image (original magnification, X40) shows that the stent struts crossing the orifice of the lumbar artery are covered with thick neointima, and the lumbar artery orifice becomes narrow.
Representative SEM findings (case 3). SEM image (original magnification, X40) shows that the ostium of the lumbar artery has luminal narrowing due to a thick neointima.
Histopathologic Findings
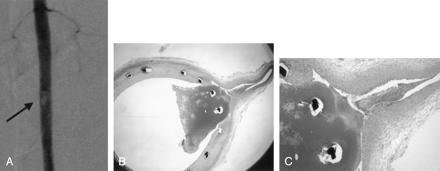
Histopathologic evaluation was performed in one rabbit. In this rabbit, follow-up angiography showed occlusion of the lumbar artery (Fig 4A). Histopathologic findings demonstrated that the thrombus surrounded the stent strut crossing the lumbar artery ostium and extended into the lumbar artery (Fig 4B and C). This thrombus was relatively new, because the infiltration of macrophages was devoid of fibrous changes.
Case of intraluminal thrombus (case 6).
A, Follow-up angiogram demonstrates intraluminal thrombus at the L4 level and occlusion of the L4 lumbar artery. Arrow indicates intraluminal thrombus.
B and C, Microscopic analysis shows intraluminal thrombus surrounding the stent struts that cross the ostium. (B, hematoxylin-eosin staining, original magnification, X12.5. C, hematoxylin-eosin staining, original magnification, X40.)
Microscopic analysis of arteries with stents showed that the stent strut was covered with a thickened neointima. This neointima was composed mainly of proliferating smooth muscle cells (data not shown).
Discussion
Symptomatic intracranial atherosclerotic stenoses often have a poor prognosis with severe neurologic deficits, despite optimal medical treatment (1–3). Percutaneous transluminal angioplasty or stent placement is expected to be an attractive treatment for this disease (5–14). However, intracranial stent placement has not been widely used because intracranial vessels have unique characteristics, including the presence of numerous perforating arteries serving important brain tissue with limited collateral vessels. Our previous report confirmed that these perforating arteries remain patent after stent placement in normal parent arteries (15). However, it is well known that atherosclerotic vessels, in comparison to normal vessels, have different characteristics such as endothelial cell defects and smooth muscle cell hyperactivity. Thus, we speculated that a differential response of atherosclerotic vessels after stent placement might result in occlusive changes to the perforating arteries. The occlusion of side branches after coronary stent placement has also been a major concern since its inception. Several clinical reports about side-branch occlusion have already been published. Poerner et al (20) examined whether or not small- and medium-sized side branches occlude after coronary stent placement. In their clinical study, chronic side-branch occlusion occurred in 13.5% of cases. The factor most strongly contributing to side-branch occlusion was a side-branch diameter of less than 1.4 mm. However, the occlusion of neurovascular perforating arteries may result in significant neurologic deficits, despite the diameters of the perforating arteries typically being much smaller than that of the coronary artery side branches. Levy et al (21) demonstrated, with angiography, that the perforating arteries were patent after stent placement of intracranial nonatherosclerotic arteries in dogs, consistent with our previous results (15). However, there are few clinical or experimental reports of the effect on perforating arteries after stent placement for intracranial atherosclerotic arteries. In only one single-center, retrospective case review, all perforating arteries remained patent after stent placement for the atherosclerotic lesions (22).
In our study, we used the rabbit abdominal aorta and lumbar artery as a proxy for intracranial arteries and perforators. The spatial relationship between the major intracranial arteries and perforating arteries in humans was similar to that of the abdominal aorta and lumbar arteries in rabbits, as described previously (15). This study demonstrates that the response of experimental atherosclerosis-induced vessels after stent placement is quite different from that of nonatherosclerotic vessels. Although the lumbar artery was patent in five of six rabbits on follow-up angiograms, the stent struts that crossed the ostium of the lumbar artery were covered with a thick neointima in SEM findings. In one case, the lumbar artery was completely occluded due to an intraluminal thrombus. In this study, the small ostial space enclosed by the stent strut was stenotic.
Recently, more suitable stents have been developed, such as drug-eluting stents that inhibit neointimal growth (23–25). Sirolimus-coated stents could completely control the proliferation of smooth muscle cells. Tanabe et al (26), however, reported that the occlusion rate of side branches after placing sirolimus-coated stents was similar to that with uncoated stents. To achieve the objective of preserving the perforating artery, a stent that can suppress the proliferation of smooth muscle cells as well as promote the reendothelialization is the most ideal. Intracranial stent placement should be further developed as a treatment for cerebrovascular revascularization, if these stents can promote perforating artery patency.
Study Limitation and Future Direction
The main limitation of this study is that it involves a small number of stents in rabbits with experimentally induced atherosclerosis. Also, we used Multi-Link stents in this experiment, because this type of stent is composed of a very thin, flat strut. However, stent design is one factor that affects restenosis rate (17). Thus, it is necessary that various stents be tried for the fate of perforating arteries after stent placement in the future.
Conclusion
Our study revealed ostial narrowing of perforating arteries following stent placement, but complete occlusion was observed in only one case. Our results suggest that despite luminal narrowing, atherosclerotic perforating arteries generally maintain their patency after stent placement. The significance of such luminal narrowing remains to be determined.
Acknowledgments
We thank the Guidant Corporation for providing the Multi-Link stents, and Prof. Yukihisa Hirao, Department of Biology, Wakayama Medical University, Wakayama, Japan, for technical support with SEM.
References
- Received April 19, 2004.
- Accepted after revision June 16, 2004.
- Copyright © American Society of Neuroradiology