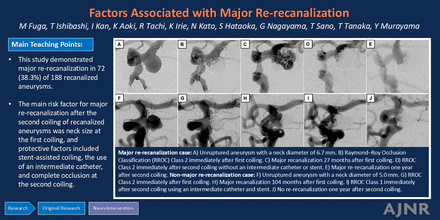
Graphical Abstract
Abstract
BACKGROUND AND PURPOSE: Second coiling for recanalized aneurysms can mitigate the risk of delayed rupture, though re-recanalization may still occur. However, factors associated with re-recanalization after second coiling for recanalized aneurysms have yet to be adequately investigated. The present study explored a large, multicenter data set accumulated over 20 years to identify factors associated with major re-recanalization after second coiling for recanalized aneurysms.
MATERIALS AND METHODS: We retrospectively reviewed 188 consecutive aneurysms in 185 patients who underwent second coiling for saccular recanalized aneurysms at 3 institutions from January 2003 to December 2023. Patients were classified into 2 groups: with major re-recanalization (R group) and without major re-recanalization (NR group). To identify factors associated with major re-recanalization, clinical, anatomic, and procedural characteristics were compared between the 2 groups by multivariate logistic regression analysis and stepwise selection.
RESULTS: During follow-up (mean, 62.3 ± 51.2 months), 72 (38.3%) of the 188 recanalized aneurysms showed major re-recanalization. In univariate analysis, compared with the NR group, the R group showed significantly larger aneurysm size, neck size, and aneurysm volume at first coiling and lower rates of stent-assisted coiling, use of an intermediate catheter (IMC), and complete occlusion at second coiling. Stepwise multivariate logistic regression analysis revealed neck size at first coiling (OR 1.18; 95% CI: 1.04–1.33) as an independent risk factor and stent-assisted coiling (OR 0.34; 95% CI: 0.15–0.79), use of an IMC (OR 0.35; 95% CI: 0.16–0.80), and complete occlusion at second coiling (OR 0.16; 95% CI: 0.033–0.70) as independent protective factors for major re-recanalization.
CONCLUSIONS: The main risk factor for major re-recanalization after second coiling of recanalized aneurysms was neck size at first coiling, and protective factors included stent-assisted coiling, use of an IMC, and complete occlusion at second coiling. Second coiling for recanalized aneurysms may reduce the risk of major re-recanalization by using a stent or IMC and achieving complete occlusion.
ABBREVIATIONS:
- GDC
- Guglielmi detachable coil
- HR
- hazard ratio
- IMC
- intermediate catheter
- IQR
- interquartile range
- LTA
- light transmission aggregometry
- LVIS
- low-profile visualized intraluminal support
- NR
- non-major re-recanalization
- R
- major re-recanalization
- ROC
- receiver operating characteristic
- RROC
- Raymond–Roy Occlusion Classification
- UCA
- unruptured cerebral aneurysm
- VER
- volume embolization ratio
SUMMARY
PREVIOUS LITERATURE:
Advances in devices such as microballoons, stents, and IMCs have made endovascular treatment of unruptured cerebral aneurysms feasible even for wide-neck and complexly shaped aneurysms, achieving low morbidity and mortality rates. Despite these advancements, recanalization and rebleeding risks remain higher than with clipping. Second coiling for recanalized aneurysms can mitigate delayed rupture risks, though re-recanalization may still occur. However, studies examining risk factors for re-recanalization after second coiling have been limited to single-center and short- to medium-term studies, and no multicenter and long-term studies have yet been conducted.
KEY FINDINGS:
This study demonstrated major re-recanalization in 72 (38.3%) of 188 recanalized aneurysms. The main risk factor for major re-recanalization after second coiling of recanalized aneurysms was neck size at first coiling, and protective factors included stent-assisted coiling, use of an IMC, and complete occlusion at second coiling.
KNOWLEDGE ADVANCEMENT:
This study suggested that a second coiling for recanalized aneurysms may reduce the risk of major re-recanalization by using a stent or IMC and achieving complete occlusion. Larger neck size at first coiling necessitates careful postoperative monitoring because of the increased risk of major re-recanalization after the second coiling.
Endovascular treatment of unruptured cerebral aneurysms (UCAs) is widely prevalent and has achieved low morbidity and mortality rates.1 Product and technique development over 2 decades have made endovascular coil treatment of wide-neck and complex-shaped aneurysms more feasible.2⇓–4
However, the higher risk of recanalization and rebleeding remains a challenge for endovascular coiling when compared with clipping.5 Recent studies in the treatment of UCAs have demonstrated that rates of recanalization and delayed rupture were 23.2%–44% and 0.94%–1.45%, respectively.6⇓⇓–9 Second coiling can be performed for recanalized aneurysms to prevent delayed rupture, but some aneurysms may nevertheless show re-recanalization.10⇓⇓–13
Factors associated with recanalization after first coiling have been shown to include age, aneurysm size, neck size, aspect ratio, aneurysm volume, aneurysm location, ruptured aneurysm, thrombosed aneurysm, incomplete occlusion, volume embolization ratio (VER), and stent use.9,14⇓⇓⇓⇓⇓–20 However, studies examining risk factors for re-recanalization after second coiling have been limited to single-center and short- to medium-term studies, and no multicenter and long-term studies have yet been conducted. Here, we investigated patients over 20 years at multiple centers to identify factors associated with major re-recanalization after second coiling for recanalized aneurysms.
MATERIALS AND METHODS
This observational study was conducted according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. The present study complied with the Declaration of Helsinki. Our institutional review board waived any requirement for informed consent because of the retrospective design.
Study Population
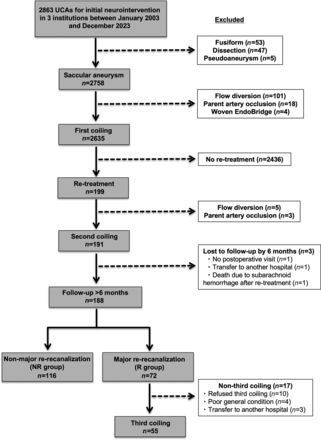
A total of 2863 consecutive first neurointerventions for UCA conducted at 3 general hospitals affiliated with the university (The Jikei University Hospital, Kashiwa Hospital, and Katsushika Medical Center) between January 2003 and December 2023 were retrospectively reviewed. At the institutions enrolled in the present study, endovascular treatment, rather than craniotomy clipping, was the first priority, regardless of the location of the aneurysm. Patients with fusiform aneurysm (n = 53), dissecting aneurysm (n = 47), or pseudoaneurysm (n = 5) were excluded so that only saccular aneurysms were included. In addition, patients with aneurysms treated by flow diversion (n=101), parent artery occlusion (n = 18), or Woven EndoBridge (MicroVention Terumo) (n = 4) were also excluded so that only coiling was included. Among aneurysms treated with first coiling for UCA, aneurysms without retreatment (n = 2436) were excluded. Aneurysms retreated with flow diversion (n = 5) or parent artery occlusion (n = 3) were also excluded, followed by aneurysms with follow-up for <6 months (n = 3). Ultimately, 188 cases with second coiling for saccular UCA in 185 patients with follow-up >6 months were included in the present study (Fig 1). Patients were classified into 2 groups: with major re-recanalization (R group) and without major re-recanalization (NR group).
Flowchart of saccular UCA selection for second coiling and subsequent classification with major re-recanalization. A total of 2863 consecutive first neurointerventions for UCAs conducted at 3 institutions between January 2003 and December 2023 were retrospectively reviewed. Patients with fusiform aneurysm (n = 53), dissecting aneurysm (n = 47), or pseudoaneurysm (n = 5) were excluded so that only saccular aneurysms were included. In addition, patients with aneurysms treated by flow diversion (n = 101), parent artery occlusion (n = 18), or Woven EndoBridge (n = 4) were also excluded so that only coiling was included. Among aneurysms treated with first coiling for UCA, aneurysms without retreatment (n = 2436) were excluded. Aneurysms retreated with flow diversion (n = 5) or parent artery occlusion (n = 3) were also excluded, followed by aneurysms with follow-up for <6 months (n = 3). Ultimately, 188 cases with second coiling for saccular UCA in 185 patients with follow-up >6 months were included in the present study. Patients were classified into 2 groups: R group and NR group.
Data Collection
The following data were obtained by retrospectively reviewing the medical records and radiologic data of these patients: age, sex, medical history (hypertension, diabetes mellitus, hyperlipidemia, polycystic kidney disease, prior stroke, and cerebral small vessel disease), smoking and drinking history, family history, aneurysm characteristics (thrombosed aneurysm, multiple aneurysms, aneurysm location, aneurysm size, neck size, aspect ratio, and aneurysm volume), endovascular technique including balloon-assisted and stent-assisted, intermediate catheter (IMC) used,4 type of coils, embolization result, VER, and complications. Cerebral small vessel disease was diagnosed if 1 or more of the following radiologic features were seen on MRI: 1) subcortical small, focal infarction, 2) diffuse white matter lesions present as white matter hyperintensities on T2WI, 3) microbleeding in the subcortical region.21 With regard to the type of coils, data were collected only for bioactive coils,22 large coils with a primary diameter of 0.014 inches or larger,23 and hydrogel coils,24 which have been previously reported to be associated with complete occlusion rates and packing density.
All aneurysms were evaluated under rotational angiography with 3D image reconstruction (The Jikei University Hospital: Artis Q Biplane, Siemens Healthineers; Kashiwa Hospital: Artis zee BA Twin Large Display, Siemens; Katsushika Medical Center: Allura Clarity FD20/10, Philips Healthcare). Aneurysm size was calculated from 3D rotational angiographic images by using NeuroVision software (Cybernet Systems), allowing automatic measurement by positioning markers on the aneurysm and parent artery.25 The aspect ratio was calculated as the ratio of the height to neck size. VER at first coiling was measured by DSA immediately after embolization. VER was calculated as follows: (volume of coil for embolization)/(volume of aneurysm) ×100 (%). Following coil insertion, the inserted coil was input into the NeuroVision software and the VER was computed.
Endovascular Procedure
All coil embolization procedures were standardized and performed under general anesthesia by or under the supervision of a certified interventional neurosurgeon. All patients received daily oral antiplatelet medication starting 1–4 weeks before coil embolization, either 100 mg aspirin alone if no stent was used or 100 mg aspirin and 75 mg clopidogrel (or 3.75 mg prasugrel) if stent placement was planned. Since August 2016, a platelet aggregation test with adenosine diphosphate was carried out by using light transmission aggregometry (LTA) (PA200C, Kyowa Hakko Bio) in patients scheduled for stent placement by the day before the procedure. The continued dose was determined based on LTA measurements on the day of coil embolization.
Following puncture of the access site, a bolus of 4000–5000 U of heparin was infused intravenously, followed by intermittent administration of 1000–2000 U of heparin to keep the baseline activated clotting time more than doubled throughout the duration of treatment.
The use of IMC depended on the discretion of the neuroendovascular surgeons, but its use was recommended in cases of vascular tortuosity. The IMCs used included DAC (Stryker Neurovascular), Cerulean (Medikit Co Ltd), Tactics (Technorat), Navien (Medtronic), Guidepost (Tokai Medical Products), AXS Vecta (Stryker Neurovascular), Asahi Fubuki (Asahi Intecc), Sofia (MicroVention Terumo), and Tracker (Target Therapeutics). All aneurysms were packed as densely as possible with coils. The type of coil used was left to the discretion of the neuroendovascular surgeons. Coils used included Guglielmi detachable coil (GDC; Target Therapeutics/Boston Scientific), Matrix2 (Stryker Neurovascular), Target (Stryker Neurovascular), Axium (Medtronic), i-ED (Kaneka Medics), Optima (Balt), HydroCoil Embolic System (MicroVention Terumo), and Galaxy (Johnson & Johnson Codman).
Adjunctive techniques such as balloon-assisted (HyperForm, Medtronic; Transform, Stryker Neurovascular; Scepter, MicroVention Terumo; or Shouryu, Kaneka Medics) and stent-assisted technique (Neuroform EZ, Stryker Neurovascular; Neuroform Atlas, Stryker Neurovascular; Enterprise1, Johnson & Johnson Codman; Enterprise2, Johnson & Johnson Codman; or low-profile visualized intraluminal support [LVIS], MicroVention Terumo) were employed for wide-neck aneurysms with neck size >4 mm or dome-to-neck ratio <2.
Postoperatively, if no stent was used, a single antiplatelet medication (aspirin) was administered for 1–4 weeks. If a stent was implanted, dual antiplatelet medications (aspirin and clopidogrel, or prasugrel) were continuously administered for ≥6 months after the procedure, then single antiplatelet medication (aspirin) was continued for at least 6 months.
Embolization Results Immediately after Treatment and Follow-Up Imaging
The embolization results immediately after treatment in the present study were assessed by using the Raymond–Roy Occlusion Classification (RROC) scores. The RROC was classified as follows: class 1, complete occlusion; class 2, residual neck; and class 3, residual aneurysm.26
Follow-up MRA (by using a ≥1.5T system) was planned at 3, 6, and 12 months after embolization, then once per year thereafter. Follow-up DSA was performed in cases where re-recanalization was suspected by MRA or at 12 months posttreatment if a stent was implanted.
Indication for the Second Coiling
Recanalization was defined as increased blood inflow compared with the condition immediately after embolization as measured by MRA or DSA. Major recanalization was defined as a confirmed increase in RROC score from 1 or 2 to 3 or an RROC score of 3 with an increase in the Meyers scale on follow-up DSA or MRA.4 Recanalization that did not meet the conditions for major recanalization was judged as minor recanalization. Indication for the second coiling was determined if major recanalization was observed after the first coiling. On the other hand, aneurysms with minor recanalization were carefully followed by MRA. All images were evaluated by 2 certified interventional neurosurgeons. In the event of discrepancies between raters, a third interventional neurosurgeon appraised the images, and consensus was reached.
Complications
Procedure-related complications were classified as ischemic and hemorrhagic; intraprocedural rupture was assigned to hemorrhagic complications. Symptomatic complications were defined as any increase of ≥1 in the mRS score from preoperative levels.
Statistical Analyses
Fisher exact test or the Mann–Whitney U test was employed to compare baseline features between the R and NR groups. Multivariate logistic regression analysis and stepwise selection were applied to identify factors associated with major re-recanalization after second coiling for recanalized aneurysms. This analysis was adjusted not only for significant factors in univariate analyses in the present study but also for previously reported risk factors for recanalization: aneurysm size, neck size, aspect ratio, aneurysm volume, aneurysm location, ruptured aneurysm, thrombosed aneurysm, incomplete occlusion, no balloon use, no stent use, or type of coils.9,14⇓⇓⇓⇓⇓–20,22⇓–24 Alterations in neck size at the time of first coiling were assessed by using receiver operating characteristic (ROC) curves and 95% CIs. The optimal cutoff was identified as the point closest to the upper left corner of the ROC curve. Statistical analyses were carried out by using R and R Commander-based Easy R (EZR) software (Saitama Medical Center, Jichi Medical School).27 Values of P < .05 were considered significant.
RESULTS
Clinical Characteristics
During follow-up (mean, 62.3 ± 51.2 months), 72 (38.3%) of the 188 recanalized aneurysms showed major re-recanalization, and 55 (29.3%) re-recanalized aneurysms underwent a third coiling. In the 17 major re-recanalization patients, the reasons for not undertaking a third coiling were as follows: 10 refused the third coiling, 4 were in poor general condition precluding a third intervention, and 3 were transferred to another hospital (Fig 1).
The clinical characteristics of patients are presented in Table 1. No significant differences were noted with respect to age, sex, smoking history, drinking history, or family history. In terms of medical history, no significant differences were found between groups in diabetes mellitus, hyperlipidemia, polycystic kidney, prior stroke, or cerebral small vessel disease. Compared with the NR group, the R group displayed a significantly higher rate of hypertension (P = .02). No significant differences between groups were identified for aneurysm characteristics including thrombosed aneurysm, multiple aneurysms, and aneurysm location (Table 1).
Baseline clinical characteristics in R and NR groups
Anatomic and Procedural Characteristics at Time of First Coiling
Anatomic and procedural characteristics were presented in Supplemental Data. Maximum aneurysm size (10.2 mm [interquartile range (IQR): 7.5–13.4 mm] versus 7.2 mm [IQR: 5.6–10.3 mm], P < .001), neck size (6.9 mm [IQR: 5.0–9.0 mm] versus 5.0 mm [IQR: 3.5–6.7 mm], P < .001), and aneurysm volume (466 mm3 [IQR: 176–1050 mm3] versus 153 mm3 [IQR: 71–343 mm3], P < .001) at time of first coiling were significantly larger in the R group than in the NR group. At first coiling, no significant differences in aspect ratio, endovascular technique, embolization result, or VER were observed between groups.
Anatomic and Procedural Characteristics and Outcome at Time of Second Coiling
Among recanalized aneurysms, the percentage of ruptured aneurysms did not differ significantly between groups. Endovascular techniques in the R and NR groups were primary coiling in 28 (39%) and 41 (35%), balloon-assisted in 7 (9.7%) and 12 (10%), double-catheter in 27 (38%) and 20 (17%), and stent-assisted in 10 (14%) and 43 (37%). Balloon-assisted coiling showed no significant difference between the 2 groups. On the other hand, stent-assisted coiling was significantly less frequently performed in the R group than in the NR group (14% versus 37%, P = .001). The types of stents in the R and NR groups were Neuroform in 6 (60%) and 38 (88%), Enterprise in 4 (40%) and 3 (7.0%), and LVIS in 0 (0%) and 2 (4.7%), respectively (Supplemental Data). With respect to the type of IMC, DAC (36%) catheter was most commonly used in the R group, Tactics (35%) catheter in the NR group, and as for the size of the IMC, 3.2–3.9 Fr was most commonly used in both groups. The most frequently positioned location of the IMC was the supraclinoid segment of the ICA (27%) in the R group and the cavernous segment of the ICA (44%) in the NR group (Supplemental Data). Use of an IMC was significantly less frequent in the R group (15%) than in the NR group (40%, P = .001) (Supplemental Data). As for the type of coils, bioactive coils were all the Matrix2, large coils with a primary diameter of 0.014 inches or larger were all the Target XL, and hydrogel coils were all the HydroCoil Embolic System. No significant difference was found between the 2 groups for large coil and hydrogel coil, but bioactive coil was used significantly more frequently in the R group than in the NR group (40% versus 22%, P = .01).
Distributions of RROC scores immediately after treatment in the R and NR groups were 2 (2.8%) and 23 (20%) for class 1, 56 (78%) and 75 (65%) for class 2, and 14 (19%) and 18 (16%) for class 3, respectively (Supplemental Data). The R group demonstrated a significantly lower percentage of RROC class 1 immediately after second coiling than the NR group (2.8% versus 20%, P = .001).
Among the second coiling for 188 recanalized aneurysms, 3 (1.6%) had perioperative complications. Ischemic complications included asymptomatic thromboembolism in 1 (0.53%) and symptomatic cerebral infarction associated with coil migration (retrieved with stent retriever) in 1 (0.53%). Hemorrhagic complications included asymptomatic cerebral hemorrhage due to hyper-response to clopidogrel of LTA value of 25 (<40) the day after second coiling in 1 (0.53%). No intraprocedural rupture occurred during the second coiling procedure for recanalized aneurysms. No significant difference in the incidence of ischemic or hemorrhagic complications was seen between the 2 groups.
Factors Associated with Major Re-Recanalization after Second Coiling for Recanalized Aneurysms
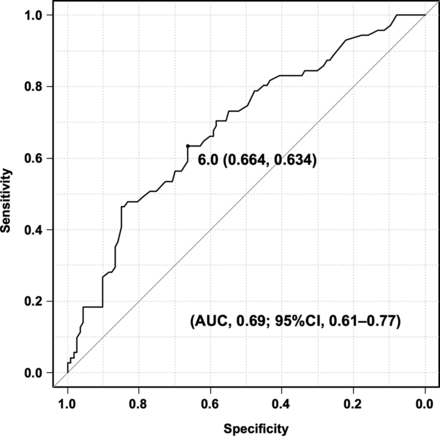
In major re-recanalization after second coiling for recanalized aneurysms, multivariate logistic regression analysis and stepwise selection identified neck size at first coiling (OR 1.18; 95% CI: 1.04–1.33) as an independent risk factor and stent-assisted coiling (OR 0.34; 95% CI: 0.15–0.79), use of an IMC (OR 0.35; 95% CI: 0.16–0.80), and RROC class 1 at second coiling (OR 0.16; 95% CI: 0.033–0.70) as independent protective factors (Table 2). Based on ROC curve analysis, the optimal cutoff for neck size at first coiling was 6.0 mm (sensitivity, 66.4%; specificity, 63.4%; area under the ROC curve, 0.69 [95% CI: 0.61–0.77]) (Fig 2).
Multivariate logistic regression analysis and stepwise selection of associated factors for major re-recanalization
ROC curve of the optimal cutoff for neck size at first coiling to distinguish between R and NR groups.
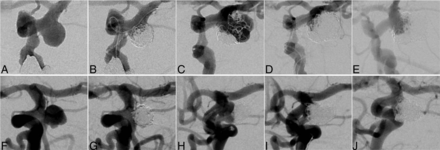
Illustrative cases of the R group and the NR group are provided in Fig 3.
Illustrative cases. R group case: A 73-year-old woman who had undergone coil embolization for an incidentally detected unruptured posterior communicating aneurysm with a maximum and neck diameter of 6.7 mm (A). An 8F balloon-guiding catheter (Merci; Stryker Neurovascular) was directed into the extracranial segment of the left ICA. The coils were inserted into the aneurysm using a double-catheter technique; no IMC, balloon, or stent was used, and the embolization result was RROC Class 2 (B). Major recanalization was discovered 27 months after the initial treatment (C). For the second coiling, 2 microcatheters (both Excelsior SL-10; Stryker Neurovascular) were navigated into the aneurysm for coil embolization. The coils were inserted into the aneurysm using a double-catheter technique and no IMC or stent was used. Ultimately, the coil embolization resulted in RROC Class 2 (D). No postoperative complications were noted, but major re-recanalization occurred 1 year after second coiling (E). NR group case: A 63-year-old woman who had undergone coil embolization for an incidentally detected unruptured cerebral aneurysm at the origin of the fetal posterior cerebral artery with a maximum diameter of 6.7 mm and a neck diameter of 5.0 mm (F). The coils were inserted into the aneurysm using a primary coiling; no IMC, balloon, or stent was used, and the embolization result was RROC Class 2 (G). Major recanalization was discovered 104 months after the initial treatment (H). A 6F guiding sheath (Asahi Fubuki; Asahi Intecc) was directed into the extracranial segment of the left ICA. A 3.2F Tactics catheter as the IMC was then guided coaxially to the supraclinoid segment of the left ICA. One microcatheter (Phenom 17; Medtronic) was navigated from the Tactics catheter into the aneurysm for coil embolization, and the other microcatheter (Excelsior SL-10) was directed into the left fetal posterior cerebral artery for stent placement. A Neuroform Atlas (Stryker Neurovascular) stent was placed from the left fetal posterior cerebral artery to the supraclinoid segment of the left ICA. Finally, the coil embolization achieved RROC Class 1 (I). No postoperative complications were noted, and follow-up DSA 1 year after the second coiling showed no re-recanalization (J). Subsequently, at 42 months, the patient remained without re-recanalization (image not shown).
DISCUSSION
In the present study, multivariate analysis identified neck size at first coiling as a risk factor for major re-recanalization after second coiling for recanalized aneurysms, while stent-assisted coiling, use of an IMC, and RROC class 1 at second coiling were protective factors. Second coiling is an imperative measure because major recanalization can rupture and subsequently result in subarachnoid hemorrhage.7,8
Risk Factors for Re-Recanalization
Second coiling for recanalized aneurysms has been associated with a higher risk of recanalization than first coiling.10⇓⇓–13,28 A review of the literature indicated that risk factors for re-recanalization have been identified as large aneurysm, wide-neck aneurysm, posterior circulation aneurysm, incomplete occlusion at second coiling, and history of autosomal dominant polycystic kidney disease (Supplemental Data).10⇓⇓–13 Cho et al10 conducted second coiling in 162 patients with a total of 197 recanalized aneurysms. During follow-up (mean, 26.0 ± 18.0 months), major re-recanalization was observed in 59 aneurysms (34.3%). Multiple logistic regression analysis independently identified the posterior circulation, large aneurysm at first coiling, and sub-total occlusion at second coiling as risk factors for major re-recanalization. They highlighted that in terms of location, aneurysms in the posterior circulation accounted for 60% of major re-recanalization after second coiling. Lee et al11 performed second coiling on a total of 133 recanalized aneurysms in 129 patients. At 6 months after second coiling, re-recanalization was found in 47 aneurysms (35.3%). Multiple logistic regression analysis revealed large aneurysm (>7 mm) at first coiling, location in the posterior circulation, and incomplete occlusion at second coiling as risk factors for re-recanalization. Bae et al13 carried out second coiling on a total of 310 recanalized aneurysms in 308 patients. During follow-up (mean, 40.2 ± 33.0 months), major re-recanalization developed in 87 aneurysms (28.1%). Multivariable Cox regression analysis indicated neck size at first coiling and autosomal dominant polycystic kidney disease as risk factors for re-recanalization. All the above findings were from short- to medium-term imaging follow-up surveys from only a single institution. The present study conducted follow-up at multiple centers over an extended period (mean, 62.3 ± 51.2 months) and replicated large neck size at first coiling as a significant risk factor for major re-recanalization, as reported by Bae et al.13 Based on the results from ROC curve analysis, more careful follow-up may be necessary after second coiling of aneurysms with neck sizes >6 mm at first coiling because of the increased likelihood of major re-recanalization. A possible mechanism that increases the risk of re-recanalization in wide-neck aneurysms can be coil compaction caused by blood flow coming into contact with the coil in the transverse plane of the aneurysm neck.29,30 Luo et al31 previously demonstrated that pressure from high-velocity blood flow may enhance the likelihood of coil compaction, especially in wide-neck aneurysms.
On the other hand, unlike previous studies,10,11 posterior circulation aneurysms were not a significant risk factor in the present study. Ferns et al32 speculated that selection bias may have influenced the high number of recanalizations involving posterior circulation aneurysms. They found that among anterior circulation aneurysms, all aneurysms that were anatomically unfavorable for endovascular treatment were sent for clipping via craniotomy. Meanwhile, among posterior circulation aneurysms, even anatomically unfavorable aneurysms were treated endovascularly.32 Such a background may have led to the high number of re-recanalizations seen among posterior circulation aneurysms. Endovascular treatment was selected as the first priority at our institutions enrolled in the present study, regardless of aneurysms located in the anterior circulation that were anatomically unfavorable for endovascular surgery. This may have led to the lack of significant difference seen for posterior circulation aneurysms in the present study.
Protective Factors for Re-Recanalization
Factors protective against re-recanalization have previously been demonstrated to include stent implantation and complete occlusion or residual neck at second coiling (Supplemental Data).10,12,13 A multiple logistic regression analysis by Cho et al10 found that placement of a stent for recanalized aneurysm at second coiling can prevent major re-recanalization during follow-up (mean, 26.0 ± 18.0 months) (OR 0.226; 95% CI: 0.088–0.581; P = .002). A multivariable Cox regression analysis by Bae et al13 showed that stent implantation (hazard ratio [HR] 0.59; 95% CI: 0.36–0.97; P = .038) and complete occlusion or residual neck at second coiling (HR 0.54; 95% CI: 0.33–0.88; P = .012) can prevent re-recanalization. The present study identified stent-assisted coiling, use of an IMC, and complete occlusion at second coiling as protective factors against re-recanalization. One possible mechanism by which the stent was a protective factor is that the scaffolding provided by the stent strut would allow for stability of the microcatheter and dense coil packing, especially around the aneurysmal neck. Considering that a wide-neck aneurysm was a significant risk factor for major re-recanalization after second coiling, the ability to achieve complete occlusion, including around the neck, by using a stent may have been a protective factor reducing major re-recanalization.33 In addition, the deployed stent can also serve as a scaffold for neointima-forming cells to completely obliterate the aneurysmal orifice.34,35 Second coiling under stent assistance for recanalized aneurysms may reduce the risk of major re-recanalization by achieving complete occlusion and promoting neointima formation.
Further, in the present study, the use of IMC was newly revealed to reduce major re-recanalization. In coil embolization for cerebral aneurysms, The IMC has the potential to increase the complete occlusion rate and packing density by improving the maneuverability and stability of the microcatheter. Our institutions previously reported a study that retrospectively investigated the efficacy and safety of IMC in coil embolization of 2430 consecutive saccular UCA aneurysms (2259 patients) by using a propensity score–matched analysis. The IMC group demonstrated significantly higher rates of RROC class 1 immediately after treatment (30.0% versus 20.8%, P = .003) and at 6 months (28.8% versus 20.0%, P = .004) as well as VER (27.2% [SD: 6.5%] versus 25.9% [SD: 6.2%], P = .003) compared with the non-IMC group, and there was no significant difference in the incidence of complications between the 2 groups.4 The IMC may be considered for more routine incorporation into the second coiling for recanalized aneurysms because coil embolization by using IMC may reduce the risk of major re-recanalization by increasing the complete occlusion rate and packing density.
Balloon-assisted techniques may increase the complete occlusion rate in coil embolization for cerebral aneurysms.2 In addition, regarding the bioactive coils, Murayama et al22 indicated that embolization by using Matrix coils (Stryker Neurovascular), which feature a platinum core coated with a bioactive, bioabsorbable polymer (polyglycolic acid/lactide), results in a lower packing density compared with embolization with GDCs. This difference was attributed to the increased friction encountered during the delivery of the coils. Complete occlusion rate and packing density have previously been shown to be associated with recanalization in coil embolization for cerebral aneurysms.14,36 However, the multivariate analysis in the present study did not identify balloon-assisted technique and bioactive coils as an independent factor associated with re-recanalization.
Given that larger neck size was a significant risk factor for re-recanalization in the present study, flow diversion may be more appropriate than coiling for recanalized aneurysms to convert the hemodynamics around the neck area. Several researchers have indicated the safety and efficacy of flow diversion for recanalized aneurysms.37⇓⇓–40 The complete occlusion rate after flow diversion for recanalized aneurysms is relatively high, ranging from 60% to 85.1%.37⇓⇓–40 The most attractive aspect of its treatment is that once complete occlusion has been accomplished, the risk of subsequent recanalization may be low.41 However, patients with recanalized aneurysms with prior stent implantation had a significantly lower rate of complete occlusion by flow diversion than those without prior stent implantation (55.6% versus 80.4%, P = .036).42 Furthermore, high rates of procedure-related complications (17.2%) and permanent morbidity (6.9%) have been noted.38 Bae et al13 encountered delayed ischemic complications associated with stent occlusion in 1 (16.7%) of 6 cases of recanalized aneurysms treated with the Pipeline Embolization Device. Studies on long-term outcomes of flow diversion for recanalized aneurysms are also still scarce. Based on the above, flow diversion may be a promising treatment for recanalized aneurysms, but there are still several issues to be resolved for flow diversion for recanalized aneurysms, including a lower rate of complete occlusion when a stent was implanted at first coiling, a higher incidence of complications, and long-term outcomes that are still unknown. The efficacy and safety of flow diversion for recanalized aneurysms should be investigated in the future.
Limitations
Several limitations to the present study need to be kept in mind and caution should be taken when interpreting the results. First, VER could not be measured at second coiling. Lower VER has previously been identified as a risk factor for recanalization at first coiling of cerebral aneurysms.14 However, for second coiling, the volume of space in the recanalized area was difficult to measure accurately because of the previously inserted coils.43 The development of imaging equipment and software capable of accurately measuring the volume of recanalized aneurysm is desirable in the future.
Second, the follow-up period had a relatively high variability, with a mean of 62.3 ± 51.2 months. Recanalized aneurysms with a longer follow-up period could have had a higher likelihood of re-recanalization because recanalization has been described as a time-dependent process.44 However, there was no significant difference between the 2 groups with and without major re-recanalization in terms of follow-up period, so the impact on the results of the present study would have been minimal.
Finally, this was a nonrandomized, retrospective, observational study, so prospective studies are needed in the future to validate our findings. However, this is the first multicenter study conducted over a longer observation period (Supplemental Data). Despite those limitations, for major re-recanalization after second coiling for recanalized aneurysms, the present study showed that neck size at first coiling was a risk factor and stent-assisted coiling, use of an IMC, and complete occlusion at second coiling represented protective factors.
CONCLUSIONS
The main risk factor for major re-recanalization after second coiling of recanalized aneurysms was neck size at first coiling, and protective factors included stent-assisted coiling, use of an IMC, and complete occlusion at second coiling. Second coiling for recanalized aneurysms may reduce the risk of major re-recanalization by using a stent or IMC and achieving complete occlusion. Larger neck size at first coiling necessitates careful postoperative monitoring because of the increased risk of major re-recanalization after the second coiling.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received August 24, 2024.
- Accepted after revision December 12, 2024.
- © 2025 by American Journal of Neuroradiology