Abstract
BACKGROUND AND PURPOSE: Symptomatic cerebral hyperperfusion has an incidence of 5% after endovascular stent placement. We hypothesized that increases in cerebral blood flow (CBF) after endovascular stent placement are positively correlated with the severity of stenosis.
METHODS: We studied patients with carotid (n = 20) or vertebrobasilar (n = 3) stenosis who were undergoing endovascular stent placement. Hemispheric CBF was measured by using intra-arterial xenon-133 technique (initial slope).
RESULTS: CBF increased from 29 ± 10 to 35 ± 12 mL/100 g/min (P = .0003) at 39 ± 12 minutes (range 13–60 minutes) after endovascular stent placement. Baseline characteristics or type of anesthesia did not affect the findings. Physiologic parameters remained constant between measurements: PaCO2 was 43 ± 6 mm Hg and arterial pressure was 89 ± 16 mmHg. The degree of vascular stenosis (70% ± 13%, range, 40–99%) was not correlated with change in CBF (r2 = 0.007, P = .70) or baseline CBF (r2 = 0.005, P = .31).
CONCLUSION: CBF increased by 21% ± 10% after treatment in the absence of clinical symptoms and without intracranial hemorrhage. Modest increases in CBF were common after endovascular revascularization. However, the increased CBF appeared to be unrelated to the degree of vascular stenosis, suggesting a relationship to availability of collateral flow pathways or a neurogenic influence.
Vascular stenoses of the head and neck are significant risk factors for stroke. Percutaneous transluminal angioplasty and endovascular stent placement are potential treatments for high-grade and symptomatic stenoses. Complications of these procedures include stroke from distal cerebral embolism or conductance vessel occlusion, possibly in conjunction with systemic hemodynamic compromise.
Cerebral hyperperfusion is a rarer cause of stroke after these revascularization procedures. Clinical symptoms are thought to result from a sudden, rapid increase in cerebral blood flow (CBF) after reperfusion of a circulatory territory exposed to long-standing decreased perfusion pressure, although the pathophysiologic characteristics of hyperperfusion syndrome remains poorly understood. Hemodynamic changes may not depend on degree of stenosis alone (1, 2) but on a combination of factors contributing to cerebral circulation. The degree of stenosis has been associated with impaired cerebral autoregulation (3) and the presence of collateral supply (4). Other associated risk factors for hyperperfusion include hypertension (5), prior infarct (6, 7), severe ipsilateral and contralateral stenosis (8, 9), clotting abnormalities (10), poor collateral circulation (11, 12), and decreased cerebrovascular reserve (13).
Hyperperfusion syndrome has been reported in patients undergoing endovascular stent placement in intracranial and extracranial vessels (14–16). Symptoms of hyperperfusion are similar to those of patients undergoing CEA. In a retrospective study, hyperperfusion syndrome occurred in 5% of patients after endovascular stent placement (14). Other than case reports (17–19), data on the effect of endovascular stent placement on cerebral perfusion are limited (20).
Intra-arterial xenon-133 measurements of CBF (21) are quantitative and can be performed intraoperatively, immediately before and after stent placement. The purpose of this study was twofold: First, we wanted to demonstrate the feasibility of rapid, repeated-measures quantitative CBF testing in the intraoperative setting of endovascular stent placement. Second, we tested the hypothesis that the degree of vascular stenosis, corresponding to the pressure drop across the stenotic vessel (22), is associated with increases in CBF after treatment. We used the intra-arterial 133Xe CBF method to quantitatively measure CBF in patients treated with endovascular stent placement and compared these changes to the preoperative baseline degree of stenosis, as determined from digital subtraction angiography.
Methods
After institutional approval was obtained, patients scheduled for endovascular stent placement for vascular stenosis provided written informed consent. The patients received stent placement under general anesthesia or intravenous sedation, which included some combination of midazolam, fentanyl, or propofol and low inspired concentrations of volatile agents. All patients underwent complete four-vessel cerebral angiography to confirm the presence of stenosis. Degree of stenosis was determined by using the North American Symptomatic Carotid Endarterectomy Trial criteria (23). For the posterior circulation, the proximal vertebral artery was used as the reference vessel to measure the degree of stenosis. Twenty-three patients were enrolled with carotid (n = 20) or vertebrobasilar (n = 3) stenoses. A stroke neurologist (N.U.K.) evaluated the patients independently during the perioperative period. Fifteen patients (65%) had a clinically symptomatic vascular lesion.
Endovascular Technique
Before the procedure, all patients were premedicated at least 72 hours with aspirin 325 mg and clopidogrel 75 mg. A transfemoral approach was used in all cases (14). After diagnostic angiography, systemic anticoagulation with heparin (70 U/kg) was given intravenously to maintain an activated clotting time 2.3–3.0 times the baseline value. After selective catheterization of the target artery was done, an 8F or 9F guide catheter (Brite Tip or Envoy; Cordis Endovascular, Miami, FL) was placed proximal to the lesion. The lesion was then crossed with a 0.014-in, 300-cm exchange guidewire (Stabilizer; Cordis Endovascular), and predilation was performed with a 3.0–3.5-mm angioplasty balloon catheter (Ninja; Cordis Endovascular). The appropriate stent device (Smart Stent, Precise Stent, BX Velocity, Cordis Endovascular; S6, S7, AVE Carotid Stent, AVE Medtronic, Santa Rosa, CA; or Mednova Carotid Stent, Abbott Laboratories, Chicago, IL) was then selected on the basis of the anatomic location and the diameter of the artery. A high-pressure, semicompliant angioplasty balloon (Ninja, Cordis Endovascular) was used to postdilate the stent to achieve more than 90% luminal diameter in most cases. The stent site and distal cerebral vasculature were angiographically evaluated after stent deployment. Percutaneous suture ligation of the arteriotomy site was performed by using the Perclose device (Abbott Laboratories).
Each patient was closely monitored in the intensive care unit for at least 24 hours after the procedure. Blood pressure parameters were individually determined with consideration to baseline blood pressure. In general, antihypertensive medications were used to maintain systolic blood pressures of 160 mm Hg or lower and a diastolic blood pressure of 100 mm Hg or lower. Heparin therapy was continued for at least 12 hours. Each patient was treated after the procedure with daily aspirin 325 mg and clopidogrel 75 mg.
CBF Measurements
After we positioned the guiding catheter in the neck, we determined the CBF by using the intra-arterial 133Xe injection technique, as previously described (21, 24–27). Bolus intra-arterial injection of 133Xe results in an instantaneous input function, avoiding the need to determine arterial concentration or deconvolution analysis of the washout curve. Briefly, two tungsten-collimated, cadmium telluride scintillation detectors from a commercial CBF collection system (Carolina Medical, King, NC) were placed on the patient’s scalp over the middle cerebral artery for anterior circulation lesions or in the posterior cerebral artery territory for posterior circulation lesions. Detector placement was confirmed by injecting contrast material during fluoroscopy.
A compact bolus of 133Xe (1–2 mCi in 1.0 mL) dissolved in saline was injected through the guiding catheter and then rapidly flushed with a 5–10 mL bolus of normal saline. Washout was recorded under stable physiologic conditions for 3 minutes. CBF was calculated by using the initial slope method, with data collected between 20–80 seconds of tracer washout, giving a value weighted toward gray matter and expressed in milliliters per 100 g of brain tissue per minute. Washout curves were individually inspected for artifact and curve fit.
Baseline hematocrit level, mean arterial pressure (MAP), heart rate, and arterial carbon dioxide partial pressure (PaCO2) were concurrently obtained with each CBF measurement. Cerebrovascular resistance was calculated by dividing MAP by CBF.
Descriptive statistics were expressed as the mean ± SD. Physiologic data and patient characteristics were analyzed by using a paired t test. Scattergrams were constructed and regression analysis performed to determine the effect of severity of stenosis on hyperperfusion after endovascular stent placement. Covariates included patient age, sex, ethnicity, presence of symptoms, and physiologic data. CBF data were reported as absolute values or percent change relative to pretreatment CBF measurements. Statistical analysis was performed by using StatView 5.0 (SAS Institute, Cary, NC).
Results
No technical or neurologic complications occurred in the 23 patients who underwent 133Xe CBF measurements before and after endovascular stent placement. (Table 1)presents the clinical and demographic data, and Table 2 shows the physiologic changes between measurements. Heart rate, blood pressure, PaCO2, and depth of anesthesia were stable between measurements. No patients had clinical symptoms after the procedure, such as headache, stroke, or TIA, attributable to hyperperfusion syndrome.
Summary of patient characteristics
Physiologic data
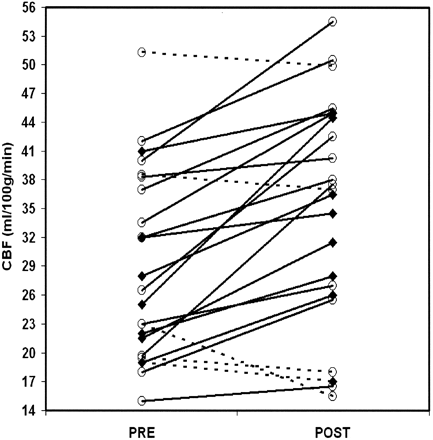
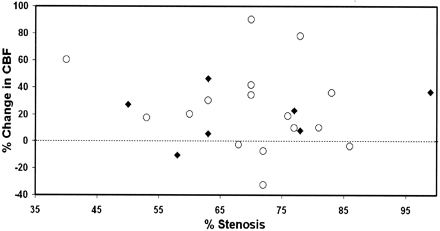
CBF increased from 29 ± 10 to 35 ± 12 mL/100 g/min (P = .0003) after endovascular treatment (Fig 1). The observed increase in CBF remained significant after multivariate analysis, with adjustment for physiologic parameters and patient characteristics. However, we found no association between severity of vascular stenosis and increase in CBF (r2 = 0.007; P = .70) or baseline CBF (r2 = 0.005; P = .31) (Fig 2). We also noted no effect of baseline characteristics or type of anesthesia. Findings in symptomatic patients and asymptomatic patients did not differ.
Individual changes in CBF before and after treatment. Solid lines represent increase in CBF; dashed lines, decrease in CBF after the procedure; circles, asymptomatic patients; and diamonds, symptomatic patients.
Percentage change in CBF as a function of degree of stenosis before endovascular stenting. Circles indicate symptomatic patients; and diamonds, symptomatic patients.
Discussion
We report what we believe the first demonstration of the acute changes in quantitative hemispheric CBF measured immediately before and after endovascular stent placement. Similar to the case for CEA, increases in cerebral perfusion seem to be a common and rarely significant event, rather than a rare symptomatic event. We could not demonstrate a relationship to the degree of preoperative stenosis.
We observed a mean 21% increase in ipsilateral CBF in the absence of clinical symptoms that was similar to the extent of increases reported after CEA (6, 13). For example, Schroeder et al (6) found that CBF increased by a median of 37% in the ipsilateral cerebral hemisphere and 33% in the contralateral hemisphere within the first postoperative day.
In patients with severe stenosis, cerebral autoregulation may be impaired, as evidenced by decreased cerebrovascular reactivity (3). Although we did not measure cerebrovascular reactivity in our patients, we thought that impaired autoregulation was most likely to occur in vascular territories distal to a marked stenosis. However, we did not find that the degree of vascular stenosis was predictive of CBF increases after treatment. At least two possible explanations may account for this observation. First, the severity of stenosis may not adequately reflect the extent of collateral perfusion pressure, which is available to the distal circulation (28). In particular, the development of a vascular stenosis due to atheromatous disease might be expected to stimulate the development or enlargement of collateral pathways. Our patient cohort had no evidence of severe strokes, and it may represent a population with better collateral supply that maintained collateral perfusion pressure (13, 22, 29). Future studies should assess the presence of collateral flow pathways and vasomotor reserve and their effect on CBF increases after endovascular stent placement (13, 22, 29). Given the evidence gleaned from CEA, extent of collateral flow and vasomotor reserve seem likely to have an association in the setting of endovascular treatments. Second, the presence of hyperperfusion may be unrelated to hemodynamic factors and may involve other neuroeffectors. The autonomic nervous system is perturbed in the postoperative period after endovascular stent placement (30), and it may contribute, through some mechanism, to the observed changes in cerebral hemodynamics (12). Such changes could involve the release of vasoactive neuropeptides involved in autoregulation. For example, animal models have demonstrated that the hyperemic response to global ischemia can be attenuated by ablation of the trigeminal ganglion (12, 31). Meyer et al (32) found surges in extracellular norepinephrine after repressurization from obliteration of chronic carotid-jugular fistulas in rodents. Intravascular release of substances that can directly influence the neurovascular unit is the object of increasing interest in the mechanism of stroke injury (33). It is important to note that the increases in perfusion after CEA appear to be bilateral (6, 13, 34); these are difficult to explain on a purely hemodynamic basis.
Our study had several limitations. We report a small sample, the findings from which can serve only as proof of principle that CBF modestly increases after endovascular stent placement; we did not encounter a wide-enough range of CBF changes to correlate them with clinical sequelae. Because of the spatial limitations of hemispheric monitoring of CBF (2–3 cm3 of tissue per detector), we may have missed hyperemic regions distant from the area of sampling. Further, smaller hyperemic regions may have been missed because of the partial volume effect inherent to our method. Such sampling errors, however, would have caused us to underestimate perfusion changes. The intra-arterial injection delivers tracer to the volume of interest, and the washout curve reflects the sum of all flow at that volume, including collateral flow. The amount of collateral flow is reflected in the CBF measurement, but we are unable to determine the extent of this contribution.
All studies that examine CBF in the absence of a concomitant measure of metabolic rate make the underlying assumption that flow is coupled to metabolism, with hyperemia defined as increase in flow in excess of metabolic need. We could not compare our intraoperative values with a baseline anesthetized value; on the basis of previous results, our CBF values are consistent with the age and anesthetic management of the study sample (21, 24–27). Finally, we could not document the time course of our observations; it is not known how long the described alterations in CBF persist. We speculate that the duration of the increases are similar to those changes seen after CEA, which appear to last at least several days (6, 13). Despite these limitations, the intra-arterial 133Xe technique appears to be a feasible intraoperative method for endovascular stent placement, and it offers the means to robustly estimate changes in perfusion in a rapid and repeated-measures fashion.
Whether modest hyperemia, to the extent we describe in this report, predisposes a person to hyperperfusion syndrome in the postoperative period is unknown. A direct application of intraoperative CBF monitoring may allow us to select patients for strict blood pressure control after the procedure. A goal of postoperative blood pressure control is to prevent pressure-passive changes in CBF that could lead to hyperperfusion-related complications. Accordingly, our results suggest that strict control of blood pressure may have a physiologic basis in this setting, even in the absence of previously described risk factors for hyperperfusion syndrome.
Quantitative hemodynamic measurements obtained before and after treatment may provide insight into the pathophysiologic characteristics of stroke in the setting of vascular stenoses. The severity of stenosis alone does not appear to be correlated with changes in brain perfusion in both symptomatic and asymptomatic cases. Use of perfusion data may allow more accurate assessment of stroke risk and improve selection of patients who may benefit from revascularization procedures.
Conclusion
Rapid repeated-measures monitoring of intraoperative CBF is feasible in the setting of endovascular stent placement, and modest increases in CBF are common after endovascular revascularization. However, increases in CBF appeared to be unrelated to the degree of vascular stenosis, suggesting a relationship to availability of collateral perfusion pressure, or alternatively, a neurogenic influence. Our findings suggest that it is difficult to predict which patients will develop the hyperperfusion syndrome on the basis of the degree of vascular stenosis alone.
Acknowledgments
The authors gratefully acknowledge the contribution of Nancy Quinnine, RN, Louis Juravsky, MD, Sean Cullen, MD, and James Mick, MD, for their assistance in conducting these studies; John Huberty, PhD, for his assistance in the preparation of the radiopharmaceuticals; Tomoki Hashimoto, MD, Mary Nelson Tran, PhD, Carroll Schreibman, and Broderick Belenson, for their preparation of the manuscript; and the other members of the UCSF Brain Arteriovenous Malformation Study Project (http://avm.ucsf.edu).
Footnotes
Supported by Public Health Service grants K23 NS044014-01A1 (to N.K.) and RO1 NS27713, K24 NS02091 (to W.L.Y.).
References
- Received February 9, 2004.
- Accepted after revision June 16, 2004.
- Copyright © American Society of Neuroradiology