Abstract
BACKGROUND AND PURPOSE: Cushing disease is typically caused by a pituitary adenoma that frequently is small and challenging to detect on conventional MR imaging. High-field-strength 7T MR imaging can leverage increased SNR and contrast-to-noise ratios compared with lower-field-strength MR imaging to help identify small pituitary lesions. We aimed to describe our institutional experience with 7T MR imaging in patients with Cushing disease and perform a review of the literature.
MATERIALS AND METHODS: We performed a retrospective analysis of 7T MR imaging findings in patients with pathology-proved Cushing disease from a single institution, followed by a review of the literature on 7T MR imaging for Cushing disease.
RESULTS: Our institutional experience identified Cushing adenomas in 10/13 (76.9%) patients on 7T; however, only 5/13 (38.5%) lesions were discrete. Overall, the imaging protocols used were heterogeneous in terms of contrast dose as well as type of postcontrast T1-weighted sequences (dynamic, 2D versus 3D, and type of 3D sequence). From our institutional data, specific postgadolinium T1-weighted sequences were helpful in identifying a surgical lesion as follows: dynamic contrast-enhanced, 2/7 (28.6%); 2D FSE, 4/8 (50%); 3D sampling perfection with application-optimized contrasts by using different flip angle evolution (SPACE), 5/6 (83.3%); and 3D MPRAGE, 8/11 (72.7%). The literature review identified Cushing adenomas in 31/33 (93.9%) patients on 7T.
CONCLUSIONS: 7T MR imaging for pituitary lesion localization in Cushing disease is a new technique with imaging protocols that vary widely. Further comparative research is needed to identify the optimal imaging technique as well as assess the benefit of 7T over lower-field-strength MR imaging.
ABBREVIATIONS:
- DCE
- dynamic contrast-enhanced
- SPACE
- sampling perfection with application-optimized contrasts by using different flip angle evolution
- VIBE
- volumetric interpolated brain examination
SUMMARY
PREVIOUS LITERATURE:
Prior reports have described high sensitivity of 7T MRI for the detection of corticotroph pituitary adenoma in Cushing disease, but in small numbers. The existing literature describes heterogeneous imaging protocols that vary in terms of contrast dose, use of dynamic contrast enhanced imaging, and 2D versus 3D post contrast imaging.
KEY FINDINGS:
Our 7T institutional data identified pituitary lesions in 10/13 patients with Cushing disease, and half of these lesions were ill-defined and not discrete. By describing pituitary adenomas as discrete vs non-discrete, we believe the current study reflects routine practice and therefore is pertinent to clinical neuroradiology.
KNOWLEDGE ADVANCEMENT:
We summarize the current state of 7T pituitary imaging in Cushing disease based on a literature review and our institutional experience. The heterogeneity among imaging protocols calls for further evaluation with multi-center involvement to determine the ideal imaging protocol.
Adrenocorticotropic hormone–producing pituitary adenomas causing Cushing disease are often small and difficult to detect with MR imaging.1,2 Cushing disease can manifest as hypertension, diabetes mellitus, obesity, hypercoagulability, osteoporosis, mood disorders, and a plethora of other symptoms and is associated with increased mortality.3,4 Successful pituitary surgery with selective adenoma resection and subsequent biochemical remission has been associated with imaging-defined lesion localization.5 Given the gravity of this medical condition, optimizing MR imaging protocols to help identify small pituitary lesions is imperative for improving patient outcomes.1,6
Prior work has shown the benefit of 3T MR imaging over lower-field-strength 1.5T MR imaging in the detection of pituitary adenomas.7⇓-9 Increasing the magnetic field strength can provide the benefit of improved SNR and contrast-to-noise ratios.10 Clinical ultra-high-field 7T MR imaging first became FDA-approved in the United States in 2017.11 Several articles have described the use of 7T MR imaging to evaluate the pituitary gland but in small numbers, given the overall lack of availability of 7T MR imaging scanners. Some of these previous articles from other institutions have reported 100% sensitivity for identifying a pituitary adenoma in Cushing disease, while anecdotally, our experience has not been as rewarding. Herein, we present our institutional experience imaging patients with Cushing disease using ultra-high-field 7T MR imaging to add to the limited body of current literature on this topic, and we also performed a review of the literature on 7T pituitary MR imaging in Cushing disease to provide an overview of the heterogeneous nature of current 7T pituitary imaging techniques and areas for possible improvement.
MATERIALS AND METHODS
Institutional Case Series
Following institutional review board approval, we retrospectively reviewed clinical pituitary images on a 7T Magnetom Terra scanner (Siemens) from our department. Using an internal database to access cases of pathology-proved Cushing disease, we reviewed patients from 2018 to 2022 who had dedicated 7T MR imaging of the pituitary gland. Images on 7T and lower-field-strength comparison studies were independently reviewed by 2 board-certified neuroradiologists who attempted to detect a pituitary microadenoma. Detected adenomas were categorized as discrete or nondiscrete, the former having distinct margins. Lesions were identified not only by having different enhancement relative to the native pituitary gland but by T2 signal intensity relative to the native pituitary gland as well. Discrepancies were resolved with a consensus read. We recorded the following 7T MR imaging protocol details: postcontrast pulse sequence, section thickness (millimeters), and dose of IV gadolinium. All pulse sequences were FDA-approved. For all enrolled patients’ scans, a determination was made as to whether a pituitary adenoma could be visualized. If an adenoma was visible, its laterality and size were recorded along with a determination of the pulse sequence that best demonstrated the lesion. A 1Tx/32Rx Head Coil (Nova Medical) and gadobutrol (Gadavist [Bayer Schering] 1 mol/mL) was used for all 7T images. Comparison with lower-field-strength (1.5T or 3T) imaging was also reviewed in the same fashion on a different day with the readers blinded to the 7T results. Basic patient demographic information, including age and sex, were tabulated.
Literature Review
A comprehensive search of several databases from January 1, 2012, to July 19, 2023, in any language, was conducted. The databases included Ovid MEDLINE and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus. The search strategy (Online Supplemental Data) was designed and conducted by an experienced librarian with input from the principal investigator of the study. Controlled vocabulary supplemented with keywords was used to search for 7T MR imaging detection of Cushing disease. For each study, we extracted the following information: first author, year of publication, comparison MR imaging field strength, surgical pathology results, number of pathology-proved lesions that were identified on 7T MR imaging, any use of 7T dynamic contrast-enhanced (DCE) postcontrast imaging, 7T postcontrast T1-weighted sequence used, and IV gadolinium dose. Descriptive statistics were used to compare different 7T imaging protocols.
RESULTS
Institutional Case Series
Thirteen patients with Cushing disease who underwent 7T MR imaging and transsphenoidal surgical resection were included in our study. Six (46.2%) were male. The mean age was 38.6 years (range, 12–66 years). Eleven patients had lower-field-strength comparison MR imaging, and a surgical lesion was visualized in 5/8 (62.5%) patients on 3T and 1/3 (33.3%) on 1.5T. Of the 13 patients with pathology-proved corticotroph adenomas included in the study, 7T MR imaging was able to identify a pituitary lesion that corresponded, including in laterality, with a pathology-proved adenoma in 10 (76.9%) patients (Fig 1). Only 5/13 (38.5%) had discrete lesions on 7T MR imaging, while 5/13 (38.5%) had nondiscrete lesions (Fig 2). All identified lesions were hypoenhancing relative to the pituitary gland. Four lesions were identified on T2-weighted imaging with 3 lesions showing hyperintense signal and 1 lesion with hypointense signal. Consensus reads were required in three 7T studies that were agreed to be nondiscrete and one 3T study that was agreed to be nondiscrete as well.
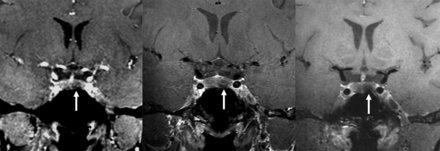
Three different types of 7T postcontrast T1-weighted imaging in a patient with a left-sided pituitary adenoma (arrows): (left to right) DCE, 2D TSE, and 3D SPACE with 1.5-, 1.5-, and 0.7-mm slice thickness, respectively.
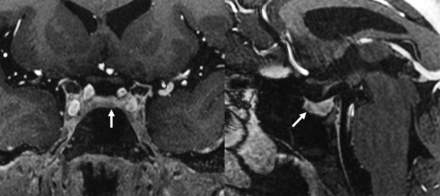
An example of 7T MR imaging showing a nondiscrete lesion (arrows) in the left paramidline pituitary gland on postcontrast MPRAGE T1-weighted imaging at 0.33 mm that was a pathology-confirmed Cushing adenoma.
Contrast doses were fixed (5 mL, 1 mmol/mL of Gadavist) for protocols including DCE and were weight-based (0.1 mL/kg, 1 mmol/mL of Gadavist) when DCE was not performed. Our contrast-enhanced imaging protocol was heterogeneous. The initial 3 patients, shortly after the FDA approval of 7T MR imaging, were imaged initially on lower-field-strength magnets, then transferred to 7T, on which only delayed postcontrast imaging was acquired (30–70 minutes after contrast injection). Even with the atypically long delay in imaging on 7T after contrast, we were able to see pituitary lesions in 2 patients.
Postcontrast T1-weighted imaging 7T sequences were also heterogeneous: 7 (53.8%) had DCE, 9 (69.2) had 2D T1-weighted imaging, 7 (53.8%) had 3D T1-weighted sampling perfection with application optimized contrast by using different flip angle evolution (SPACE sequence; Siemens), and 12 (92.3%) had 3D T1-weighted MPRAGE. One patient had nondiagnostic motion-degraded 2D imaging, 3D SPACE, and 3D MPRAGE. Of note, 5 of our patients who were imaged with 7T had already undergone a prior partial pituitary resection and underwent further imaging before a second resection.
Literature Review Patients
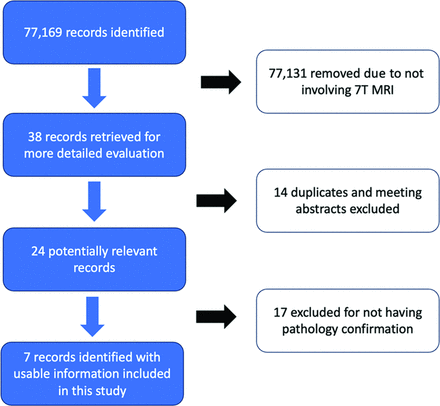
The literature review identified 33 patients with Cushing disease using 7T MR imaging (Fig 3). The patients in the study of de Rotte et al,12 in 2014, were included in the 2016 article by the same authors,13 and, therefore, were only counted once for this review. Overall, 7T MR imaging identified pathology-confirmed pituitary lesions in 31/33 (93.9%) patients. There was a large amount of variance between each MR imaging protocol, not only between each article but even within the same study at a single institution. Only 2 studies used 3D postcontrast T1-weighted imaging sequences, with the remainder using 2D sequences. One prior study used DCE imaging. The gadolinium doses also varied between weight-based and fixed-dose.
Flow chart of the search and selection of studies.
The first work on 7T pituitary MR imaging (Philips Healthcare) was performed by de Rotte et al,12 in 2014. They scanned 10 healthy volunteers and 5 patients with suspected Cushing disease. The latter patient group was included as part of a larger population that was published 2 years later.13 In total, they evaluated 16 patients with clinically and biochemically confirmed Cushing disease whose initial 1.5T MR imaging report was negative for Cushing disease or inconclusive for adenoma detection. This work had dynamic postcontrast imaging at 1.5T, but not at 7T. Pituitary lesions were identified at 7T in 13 of the 16 patients with biochemically proved Cushing disease. Only 13 of their patients underwent surgical resection. Of those, 4 patients had incomplete operative details and the laterality of the positive pathology was not known. Of the 9 patients with pathology-proved adenomas, all were identified on 7T MR imaging. Most interesting, in their study, the adenomas were hyperenhancing relative to the pituitary gland on 7T. This authorship laid the groundwork for 7T pituitary imaging while acknowledging the limitations of a long acquisition time (10 minutes 40 seconds), incomplete surgical reports to confirm the MR imaging findings in several patients, and lack of 7T DCE sequences.
Law et al14 added to this work with a case report of a patient with Cushing disease who had prior negative 1.5T and 3T MR imaging findings, and detected a microadenoma on 7T imaging. This protocol used postcontrast 3D MPRAGE but did not have DCE imaging.
Later, from the same institution as Law et al,14 Patel et al15 scanned 8 patients with pathology-confirmed Cushing disease on a Magnetom Terra 7T system (Siemens). They performed 3 types of postcontrast T1-weighted imaging but did not have dynamic postcontrast imaging in their protocol. Their 7T MR imaging studies identified lesions in all 8 patients. Seven (88%) were shown to be corticotroph adenomas, and 1 patient had Crooke hyaline change with endocrinologic remission after surgery. 3D SPACE imaging detected a lesion in all 8 patients who were included. MPRAGE postcontrast imaging was performed in 5 patients, and a lesion was detected in 4 patients. 2D TSE postcontrast T1-weighted imaging detected a lesion in 3/4 patients.
More recently, Eisenhut et al16 scanned 18 patients for suspected microadenomas with 3T and 7T MR imaging. Their 7T protocol used a 2D FLASH sequence. This was the first article to include dynamic postcontrast imaging. The authors did not use 3D thin-section, submillimeter, postcontrast imaging as was the case with prior studies. Of the 18 patients, 16 had pathology-proved adenomas; 13 of whom were patients with Cushing disease. 7T identified the lesion in 12/13 (92.3%) patients. All were seen on the T1 coronal postcontrast sequences, and 13/15 (86.7) of all microadenomas (including non-Cushing) were seen on the DCE sequences. 7T also detected 3 lesions that were not seen at 3T.
Feng et al17 reported on the surgical resection technique for a single patient with MR imaging negative for Cushing disease. Their patient underwent 3T and 7T MR imaging. Findings of both were negative; however, imaging details were not provided.
Eisenhut et al18 described their 7T MR imaging experience with pituitary macroadenomas to evaluate cavernous sinus invasion. They did not have DCE or 3D postcontrast T1-weighted imaging; however, they found the 7T image quality to be improved over 1.5/3T. One patient had Cushing disease, and the authors were able to identify the lesion on 7T. They also found improved ability to detect cavernous sinus invasion on 7T.
DISCUSSION
In the current study, we have described our institutional experience with 7T MR imaging for pituitary lesion localization in patients with Cushing disease. Additionally, we performed a literature review of the use of 7T MR imaging in Cushing disease. Exploring accurate and reliable imaging techniques is critical because lower-field-strength MR imaging can miss up to 50% of adenomas in Cushing disease.1,2 We highlight the effectiveness of using 7T over lower-field-strength MR imaging to evaluate pituitary lesions in patients with Cushing disease as well as the need for further improvement and standardization in clinical 7T MR imaging of the sella.
Both the literature review and our single-center experience demonstrate the extreme heterogeneity in pituitary MR imaging protocols. This finding is not unexpected given the recent FDA approval of clinical 7T MR imaging and the lack of consensus on optimal imaging protocols. Pituitary adenomas in Cushing disease are frequently microadenomas, which pose a substantial challenge for detection due to their small size—often only a few millimeters in diameter.19 The improved sensitivity of 3T MR imaging over 1.5T for the detection of microadenomas has previously been established.19 Therefore, many have hoped that a further increase in the magnetic field strength of MR imaging would continue to improve detection of these miniscule tumors. In our institutional patients, while we identified pathology-confirmed lesions in 10/13 (76.9%) patients, one-half of the lesions on contrast-enhanced 7T MR imaging were nondiscrete and quite subtle. Our work is the first 7T article to describe pituitary adenomas as discrete versus nondiscrete, reflecting routine practice in the reading room and is, therefore, relevant to clinical neuroradiology. Within this context, it remains unclear how the varying technical aspects of 7T pituitary imaging protocols impact this sensitivity.
DCE
Only 1 article in our literature review used DCE postcontrast imaging at 7T.16 The authors found a pathology-confirmed pituitary lesion in 12/13 patients; however, this was surpassed by their 2D FLASH postcontrast sequence that identified a pituitary lesion in all 13 patients. Prior studies on lower-field-strength MR imaging have found DCE to be superior to delayed postcontrast imaging in detecting microadenomas.19⇓-21 Our institutional cohort provided the second report of using DCE at 7T for patients with Cushing disease. The DCE sequences in our institution were low-yield, identifying a lesion in only 2/7 (28.6%) patients. However, in 1 of the 2 successful cases, DCE was the only sequence that was able to identify a pituitary lesion because the other pulse sequences were limited by motion artifacts. This result is due to the decreased acquisition time of DCE as a potential additional benefit over other conventional sequences because the latter may be more prone to motion degradation in certain patients. In the earlier articles listed in the literature review,12,13 the nondynamic 7T sequences required >10 minutes to acquire. DCE performed at 7T can repeatedly image the pituitary gland at 15- to 25-second intervals, similar to lower field strengths. It is, therefore, substantially less motion-sensitive than other pituitary pulse sequences.
Nondynamic, T1-Weighted Postcontrast Sequences
The studies in our literature review used a variety of postcontrast T1-weighted images, which reflects the overall lack of evidence regarding their comparative effectiveness. Work evaluating multiple 3D postcontrast T1-weighted sequences in brain tumors found that SPACE and volumetric interpolated brain examination (VIBE) sequences obtained higher contrast rating, contrast-to-noise ratio, and visual conspicuity ratings over MPRAGE in both gliomas and metastases.22 Specific to pituitary imaging, prior work has advocated the use of volume isotropic TSE acquisition (or VISTA),23 while others have shown the benefit of VIBE.24 Patel et al15 had similar success with different postgadolinium 3D T1-weighted pulse sequences: 88% sensitivity with SPACE and 80% with MPRAGE. In our institutional data with a small sample size, we found more success with MPRAGE over SPACE in patients in whom both sequences were used. Overall, there is a lack of data to provide strong evidence to support any specific postgadolinium T1 sequence.
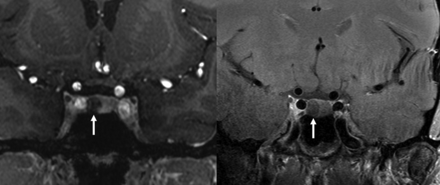
Additionally, our literature review revealed heterogeneity between the use of 3D versus 2D postcontrast imaging. 3D has the benefit of providing multiplanar reformats. Also, it typically provides increased spatial resolution when assessed by voxel-size metrics compared with 2D techniques. The latter would seem to be most beneficial in Cushing disease because small adenomas <4 mm are challenging to detect with MR imaging.19 Wang et al25 found that 3D SPACE imaging outperformed 2D imaging in defining pituitary lesions at 3T. Additional 3D sequences, including VIBE/echo-spoiled gradient echo, have been found to detect Cushing microadenomas at a higher rate than 2D spin-echo T1-weighted postcontrast images.26 At 7T field strength, Eisenhut et al16 had the largest collection of cases and good success (92.3% sensitivity) with 2D postcontrast imaging with 1.5-mm section thickness. In our institutional data, we had 8 patients with both 2D and 3D postcontrast imaging (Fig 4). In 2 patients, we saw lesions on 3D imaging that were not seen on 2D. On the other hand, we did not have any patients in whom lesions were seen only on 2D.
7T postcontrast T1-weighted imaging in a patient with a right-sided pituitary adenoma (arrows). The left image is a 3D MPRAGE sequence, while the right image is a 2D TSE sequence. The MPRAGE image demonstrates greater contrast between the normally enhancing pituitary gland compared with the hypoenhancing adenoma.
Additional Sequences
Neither our institutional patient experience nor prior studies have described the use of postcontrast T2-weighted FLAIR imaging or CISS sequences, both of which have been described as being useful at lower field strengths.24,27 CISS is a volumetric imaging technique that has been more frequently used to outline the subarachnoid spaces in the posterior fossa. Similarly, T2-weighted FLAIR imaging has some T1-weighted properties as well. In fact, it is very sensitive to low concentrations of gadolinium contrast, and has been predominantly used to identify leptomeningeal disease.28 More recently, contrast-enhanced 3D STIR FLAIR imaging was found to have increased pituitary adenoma conspicuity compared with 2D T1-weighted sequences.29 This study was performed at 3T, but a similar technique has not been described at 7T.
Gadolinium Dose
The ideal gadolinium dose for pituitary MR imaging is unknown. There are 2 standard approaches to IV contrast dosing: fixed versus weight-based. Our literature review included studies with both types of dosing. When weight-based dosing is used, standard brain MR imaging is performed with 0.1 mmol/kg of gadolinium contrast. In 2006, Bartynski et al30 used half-dose (0.05 mmol/kg), normal-dose (0.1 mmol/kg), and double-dose (0.2 mmol/kg) IV gadolinium-based contrast on a 1.5T scanner. The calculated contrast ratio between lesions and the pituitary gland were the same at each dose, but the absolute signal difference was largest at the double-dose, suggesting a theoretic benefit. Four years later, Portocarrero-Ortiz et al31 found that half-dose weight-based contrast increased the detection of adrenocorticotropic hormone–secreting pituitary adenomas in patients with Cushing disease. Prior work has shown that higher field strength MR imaging increases the effectiveness of gadolinium contrast agents, with higher lesion enhancement at 7T MR imaging using a half-dose of contrast compared with a full dose of contrast on 3T; however, this research was evaluating primary brain tumors and metastases rather than pituitary adenomas.32
Our article has several limitations. First, our literature review and institutional data have small patient numbers. This is due to clinical 7T MR imaging only recently receiving regulatory clearance in the United States and the European Union in 2017 as well as the low incidence of Cushing disease. Additionally, one of the studies13 reported 7T MR imaging results in 16 patients but had incomplete or absent pathology confirmation in 7 patients. One of the other studies14 was a single case report of a case with findings positive on 7T. Therefore, the composite sensitivity of 41/46 (89.1%) across studies should be interpreted with caution, acknowledging the limitations of the individual studies. Our study should not be interpreted as measuring the true sensitivity of 7T, as oftentimes MR imaging negative cases do not go to surgery and therefore were not included in the literature.
Given the 7T MR imaging protocol heterogeneity between and within institutions, larger numbers of patients must be studied to determine the 7T pituitary protocol with the best sensitivity and specificity. This issue could be potentially addressed with standardization of imaging protocols, larger samples sizes, and multisite collaboration. Second, as already discussed, several sequences (such as postcontrast FLAIR and CISS) that have been described as beneficial in the lower-field-strength pituitary imaging literature were not performed in any of the 7T imaging cohorts reviewed in this article. Our institutional experience has found that sphenoid sinus pneumatization limits the usefulness overall on 7T. We are in the early stages of clinical implementation of 7T imaging. 7T MR imaging cannot yet be determined to be entirely superior to 3T MR imaging in the detection of pituitary microadenomas. Rather, it should be viewed as a valuable tool that is yet to be perfected.
In addition to clarifying the role of DCE, 2D-versus-3D postcontrast imaging and IV contrast dose, future areas to further improve 7T pituitary imaging may include the addition of novel pulse sequences. Artificial intelligence–based reconstruction algorithms and hardware improvements such as increased scanner gradient performance and parallel transmit techniques may help through a combination of higher resolution, better lesion-to-background contrast, shorter acquisition times, and reduction in skull base artifacts.11 Further refinement of 7T pituitary imaging could also lead to detecting more incidental pituitary findings and, may therefore, be most beneficial in pathologies such as Cushing disease, in which surgical resection is imminent and lesion identification will directly impact patient care.
CONCLUSIONS
7T MR imaging for pituitary lesion localization in Cushing disease is a new technique with only small case series having been published and little consensus among imaging protocols for optimal evaluation of pituitary tumors. Accurate preoperative assessment of pituitary lesions in Cushing disease is paramount to successful patient outcomes. Further comparative research, possibly through multisite collaboration, is needed to identify the optimal imaging technique as well as to assess the benefit of 7T over lower-field-strength MR imaging.
Footnotes
All authors contributed to all categories established by the International Committee of Medical Journal Editors including the following: conception and design, or acquisition of data, or analysis and interpretation of data, drafting the article or revising it critically for important intellectual content, final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received December 13, 2023.
- Accepted after revision February 13, 2024.
- © 2024 by American Journal of Neuroradiology