Abstract
BACKGROUND AND PURPOSE: MR imaging is the technique of choice for patients presenting with acute loss of visual acuity with no obvious ophthalmologic cause. The goal of our study was to compare orbits contrast-enhanced 2D coronal T1WI with a whole-brain contrast-enhanced 3D (WBCE-3D) TSE T1WI at 3T for the detection of optic nerve enhancement.
MATERIALS AND METHODS: This institutional review board–approved retrospective single-center study included patients presenting with acute loss of vision who underwent 3T MR imaging from November 2014 to February 2020. Two radiologists, blinded to all data, individually assessed the presence of enhancement of the optic nerve on orbits contrast-enhanced 2D T1WI and WBCE-3D T1WI separately and in random order. A McNemar test and a Cohen κ method were used for comparing the 2 MR imaging sequences.
RESULTS: One thousand twenty-three patients (638 women and 385 men; mean age, 42 [SD, 18.3] years) were included. There was a strong concordance between WBCE-3D T1WI and orbits contrast-enhanced 2D T1WI when detecting enhancement of the optic nerve: κ = 0.87 (95% CI, 0.84–0.90). WBCE-3D T1WI was significantly more likely to detect canalicular enhancement compared with orbits contrast-enhanced 2D T1WI: 178/1023 (17.4%) versus 138/1023 (13.5%) (P < .001) and 108/1023 (10.6%) versus 90/1023 (8.8%) (P = .04), respectively. The WBCE-3D T1WI sequence detected 27/1023 (3%) instances of optic disc enhancement versus 0/1023 (0%) on orbits contrast-enhanced 2D T1WI. There were significantly fewer severe artifacts on WBCE-3D T1WI compared with orbits contrast-enhanced 2D T1WI: 68/1023 (6.6%) versus 101/1023 (9.8%) (P < .001). The median reader-reported confidence was significantly higher with coronal T1WI compared with 3D TSE T1WI: 5 (95% CI, 4–5) versus 3 (95% CI, 1–4; P < .001).
CONCLUSIONS: Our study showed that there was a strong concordance between WBCE-3D T1WI and orbits contrast-enhanced 2D T1WI when detecting enhancement of the optic nerve in patients with acute loss of visual acuity with no obvious ophthalmologic cause. WBCE-3D T1WI demonstrated higher sensitivity and specificity in diagnosing optic neuritis, particularly in cases involving the canalicular segments.
ABBREVIATIONS:
- ON
- optic neuritis
- OCE-2D
- orbits contrast-enhanced 2D
- WBCE-3D
- whole-brain contrast-enhanced 3D
Optic neuritis (ON) is an inflammatory disease of the CNS affecting the optic nerve, clinically characterized by acute vision loss associated with orbital pain and dyschromatopsia.1,2 ON can be isolated or associated with various diseases such as MS or neuromyelitis optica spectrum disorder.3,4 It is important to diagnose it correctly and accurately to adapt both management and treatment.5
Brain imaging is recommended by the international guidelines when diagnosing ON to assess any associated brain lesion related to previously mentioned etiologies.6 MR imaging is the criterion standard to diagnose ON for patients presenting with a loss of visual acuity. Conventional protocol includes fat-suppressed coronal T2WI and contrast-enhanced fat-suppressed coronal T1WI sequences covering the optic nerve along with FLAIR-weighted and contrast-enhanced T1WI covering the brain.7 Typical MR imaging features of acute ON consist of a high signal intensity of the optic nerve on T2WI sequences and enhancement, which is routinely evaluated on contrast-enhanced coronal T1WI sequences.8
However, evaluating the optic nerve using 2D sequences can be challenging due to its oblique and nonlinear course along its pathway. Additionally, certain segments, such as the intracanalicular segment, can be particularly challenging due to the orientation of the optic canal and the presence of artifacts, including magnetic susceptibility artifacts, due to its proximity of the sinuses.
Recent studies evaluated the diagnostic performance of new MR imaging sequences, such as double inversion recovery or 3D TSE black-blood T1WI, to increase the detection of signal abnormalities and enhancement of the optic nerve.9,10 Contrast-enhanced 3D TSE T1WI has the advantage of covering both the brain and the orbits. It might be accurate to detect enhancement of the optic nerve in patients with a suspected diagnosis of ON.
Therefore, the purpose of our study was to compare a whole-brain contrast-enhanced 3D (WBCE-3D) TSE T1WI versus orbits contrast-enhanced 2D (OCE-2D) coronal T1WI at 3T to detect optic nerve enhancement in patients with acute loss of vision with no obvious ophthalmologic cause.
MATERIALS AND METHODS
Study Design
This retrospective single-center study was conducted in a tertiary referral center specializing in ophthalmologic disorders. It was approved by an independent institutional review board and adhered to the tenets of the Declaration of Helsinki (CE_20200204_2_ALR, NCT04793087). Signed informed consent was waived by the institutional review board. This study follows the Standards for Reporting of Diagnostic Accuracy Studies guidelines.11
Participants
We included all patients presenting to our center with acute loss of visual acuity who underwent MR imaging from November 2014 to February 2020. Inclusion criteria were the following: 1) older than 18 years of age; 2) acute loss of vision with no obvious ophthalmologic cause; and 3) completion of brain and orbital MR imaging. Exclusion criteria were an incomplete MR imaging examination defined by the absence of either contrast-enhanced T1WI or WBCE-3D T1WI and detection of an orbital or brain abnormality explaining the vision loss, such as tumors involving the optic nerve or its sheaths or the optic pathways. One thousand twenty-three participants were ultimately included for analysis.
The selection of participants is shown in the Online Supplemental Data.
MR Imaging Protocol
All MR images were acquired with a 3T MR imaging scanner (Ingenia Elition; Philips Healthcare) using a 32-channel array head coil.
The brain MR imaging protocol was standardized in our institution, including fat-suppressed WBCE-3D T1WI covering the whole brain and fat-suppressed OCE-2D T1WI covering the whole length of the optic nerve as well as the optic chiasm, as recommended by the Magnetic Resonance Imaging in Multiple Sclerosis international consensus group.7 IV gadobutrol (Gadovist; Bayer) was administered at a concentration of 0.1 mmol/kg. Contrast-enhanced sequences were acquired >5 minutes after injection to obtain satisfactory postdelay contrast of structures.
Detailed acquisition parameters are shown in Table 1.
Detailed MR imaging acquisition parameters of OCE-2D coronal T1WI and WBCE-3D TSE T1WI
MR Imaging Analysis
WBCE-3D T1WI and OCE-2D T1WI were anonymized into 2 distinct imaging data sets. Two radiologists, 1 junior radiologist (D.P). and 1 senior neuroradiologist (F.C.) with 6 months and 15 years of experience, respectively, blinded to all data, individually read both imaging data sets in a random order to avoid establish recognition patterns. A second reading was performed 4 weeks later to evaluate intrareader agreement. Four weeks later, a consensus reading session was performed with a third senior neuroradiologist, also blinded to all data, who was specialized in ophthalmologic imaging with 10 years of experience (A.L.). This last reading was considered the reference standard for statistical analysis. All reading sessions were on a dedicated workstation with the Carestream software (Onex).
The readers assessed the following characteristics of participants’ MR images:
The primary judgment criterion was the presence of enhancement of the optic nerve or the optic chiasm, with the normal-appearing white matter serving as a reference
Secondary judgment criteria were the following:
The side of the enhancement
The location of the enhancement of the optic nerve, defined as intraorbital, canalicular, or cisterna
The presence of enhancement of the optic disc, considered distinct from the intraorbital segment
The presence of artifacts defined as follows: no artifacts, moderate artifacts not preventing the interpretation of the scan, and severe artifacts preventing any accurate interpretation of the scan
The reader-reported confidence when detecting optic nerve or optic chiasm enhancement, measured on a 5-point scale was follows: 1 corresponded to very low confidence; 2, to low; 3, to moderate; 4, to high; and 5, to very high confidence.
Statistical Analysis.
Quantitative variables were presented as mean (SD) and median (interquartile range); and categoric variables, as percentages. A McNemar test and the Cohen κ method were used for comparing the 2 MR imaging sequences.
A Wilcoxon signed-rank test was used to compare the reader-reported confidence and the presence of artifacts. All tests were 2-tailed.
Inter- and intraobserver agreement for the MR imaging reading was assessed using a weighted Cohen κ method for ordinal variables and the Cohen κ method for binary variables with a 95% confidence interval and were interpreted as follows: <0.20, none; 0.21–0.39, minimal; 0.40–0.59, weak; 0.60–0.79, moderate; 0.80–0.90, strong; and >0.9, almost perfect.
Data were analyzed by using R software (Version 4.0.3; http://www.r-project.org/).12
RESULTS
Demographics
One thousand two hundred twenty patients presented to our center with an acute loss of vision from November 2014 to February 2020. One hundred fifty-six (13%) patients were excluded due to the absence of either OCE-2D T1WI or WBCE-3D T1WI. Forty-one (3%) patients were excluded due to the presence of a tumor involving the optic nerve or its sheaths or the optic pathways on imaging (31 meningiomas, 6 pituitary macroadenomas, 2 optic nerve gliomas, 1 craniopharyngioma, and 1 occipital glioblastoma).
One thousand twenty-three patients were ultimately included in the study (638 women and 385 men; mean age, 42 [SD, 18.3] years) (Online Supplemental Data). Three hundred one of 1023 (29%) patients had a final diagnosis of acute ON.
Concordance of WBCE-3D T1WI and OCE-2D T1WI Sequences When Detecting Optic Nerve Enhancement
There was a strong concordance between WBCE-3D T1WI and OCE-2D T1WI when detecting enhancement of the optic nerve: κ = 0.87 (95% CI, 0.84-0.90).
WBCE-3D T1WI was significantly more likely to detect canalicular enhancement compared with OCE-2D T1WI: 108/1023 (10.6%) versus 90/1023 (8.8%) (P = .04), respectively (Fig 1). WBCE-3D T1WI detected 27/1023 (3%) optic disc enhancements versus 0/1023 (0%) on OCE-2D T1WI.
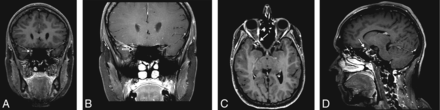
A 21-year-old woman presenting with acute vision loss and orbital pain of the right eye. WBCE-3D TSE T1WI reformatted in the coronal plane (A) shows enhancement of the intraorbital segment of the right optic nerve at the orbital apex (white arrow), whereas no optic nerve enhancement was detected on OCE-2D coronal T1WI (black arrow) (B). The WBCE-3D T1WI sequence reformatted in the axial (C) and sagittal (D) planes confirming and precisely localizing the enhancement of the optic nerve.
Three patients had optic nerve enhancement that was observable on the OCE-2D T1WI sequence but not on the WBCE-3D T1WI sequence.
Detailed results are shown in Table 2.
Comparison of WBCE-3D TSE T1WI and OCE-2D coronal T1WI
Diagnostic Performance of WBCE-3D T1WI and OCE-2D T1WI Sequences When Detecting ON
Two hundred thirty-five of 301 (78%) patients with a final diagnosis of ON had contrast enhancement of the optic nerve on WBCE-3D T1WI versus 205/301 (68%) on OCE-2D T1WI (Fig 2).
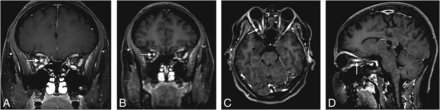
A 27-year-old woman presenting with acute vision loss and orbital pain of the right eye. OCE-2D coronal T1WI (A) shows enhancement of the intraorbital segment of the right optic nerve (arrow), also seen on the WBCE-3D TSE T1WI reformatted in the coronal (B), axial (C), and sagittal (D) planes.
The sensitivity, specificity, positive predictive value, and negative predictive value of WBCE-3D T1WI and OCE-2D T1WI when diagnosing ON were 0.78 versus 0.68, 0.97 versus 0.92, 0.92 versus 0.77, and 0.91 versus 0.87, respectively (Fig 3).
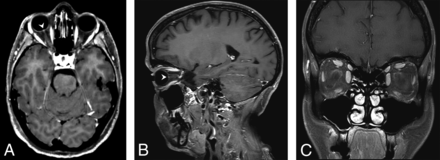
A 53-year-old woman presenting with acute vision loss of the right eye. WBCE-3D TSE T1WI reformatted in the axial plane (A) shows enhancement of the right optic disc (arrowhead). The WBCE-3D T1WI sequence reformatted in the sagittal plane (B), confirming the enhancement. No enhancement was detected on OCE-2D coronal T1WI (C).
Presence of Artifacts
There were significantly fewer severe artifacts on WBCE-3D T1WI compared with OCE-2D T1WI: 68/1023 (6.6%) versus 101/1023 (9.8%) (P < .001).
Reader-Reported Confidence
Median reader-reported confidence was significantly higher with OCE-2D T1WI compared with WBCE-3D T1WI: 5 (95% CI, 4–5) versus 3 (95% CI, 1–4) (P < .001).
Detailed results are shown in Table 2.
Interobserver and Intraobserver Agreement
The interobserver agreement when detecting optic nerve enhancement was strong on OCE-2D T1WI versus moderate on WBCE-3D T1WI: κ = 0.80 (95% CI, 0.75–0.85) versus 0.65 (95% CI, 0.60–0.71). The intraobserver agreement when detecting optic nerve enhancement was moderate on OCE-2D T1WI and almost perfect on WBCE-3D T1WI: κ = 0.75 (95% CI, 0.70–0.80) and κ = 1 (95% CI, 1–1).
Detailed inter- and intraobserver agreement is reported in Table 3.
Inter- and intraobserver agreement for a WBCE-3D TSE T1WI and OCE-2D coronal T1WI when detecting optic nerve or optic chiasm enhancement
DISCUSSION
Our study showed a strong concordance between 3D WBCE-3D TSE T1WI and OCE-2D coronal T1WI when detecting enhancement of the optic nerve in patients with acute loss of visual acuity with no obvious ophthalmologic cause. WBCE-3D T1WI showed fewer severe artifacts compared with OCE-2D T1WI and could detect optic disc enhancement.
To the best of our knowledge, our study is the largest one comparing the diagnostic concordance of these 2 sequences in clinical practice.
We showed that WBCE-3D T1WI had a higher detection rate when detecting optic nerve enhancement in its canalicular segments. Our study is in concordance with existing literature, especially with the study of Riederer et al9 comparing a 3D T1WI black-blood sequence with a 3D T1WI MPRAGE sequence. In that study, 70% of the patients diagnosed with ON had optic nerve enhancement, with a sensitivity of 70% and specificity of 90% for detecting optic nerve contrast enhancement. Canalicular segments are considered difficult to assess using coronal T1WI because of the orientation of the optic canal, the reduced contrast between the optic nerve and the surrounding hypointense bones, and the presence of artifacts due to the proximity of the sinuses. The higher detection rate of WBCE-3D T1WI might be due to the ability of multiplanar reconstructions used in 3D sequences, improving both detection and localization of the enhancement. 3D reformatting is known to increase accuracy by overcoming the partial volume effect compared with 2D sequences.13 The increase might also be due to the presence of fewer artifacts, which were significantly lower using the WBCE-3D T1WI compared with OCE-2D T1WI. This result is in line with the better clarity of the optic nerve and the higher visual enhancement of the optic nerve with 3D Cube T1WI (GE Healthcare) compared with 2D T1WI as reported by a recent study comparing these 2 sequences when diagnosing contrast-enhancing lesions of the optic nerve.14
WBCE-3D T1WI has several advantages over OCE-2D T1WI. It allows analysis of the whole brain to detect white matter lesions with excellent diagnostic performance.15 It can analyze the optic disc and thus detect optic disc enhancement, which is not feasible with OCE-2D T1WI. In our study, 27 patients had enhancement of the optic disc on WBCE-3D T1WI, which could not be assessed on OCE-2D T1WI. Fat saturation slightly reduces the SNR of the WBCE-3D T1WI. However, fat saturation prevents certain folding artifacts, especially those of fat in the cerebral parenchyma, particularly in patients who have slight movement. This is our routine sequence for analyzing cerebral parenchyma after contrast injection.
Most interesting, self-reported confidence was significantly higher with OCE-2D T1WI compared with WBCE-3D T1WI. Better confidence might be explained by the radiologist’s habit of looking for optic nerve enhancement on dedicated OCE-2D T1WI rather than on WBCE-3D T1WI in routine clinical practice.
Despite the strong concordance between WBCE-3D T1WI and OCE-2D T1WI, our study advocates the use of both sequences when performing MR imaging in patients with a suspected diagnosis of ON instead of replacing OCE-2D T1WI with WBCE-3D T1WI, because both sequences have strengths and weaknesses. A combination of both sequences might increase the detection rate of optic nerve enhancement and the confidence of readers, especially in cases of artifacts, such as susceptibility or motion artifacts involving only 1 sequence.
Our findings emphasize the importance for radiologists to look for optic nerve enhancement on a WBCE-3D T1WI sequence during the diagnosis or follow-up of patients with inflammatory brain lesions, in order to diagnose asymptomatic ON, which is reported to occur in up to 50% of patients with MS.16 Because there are no official guidelines regarding the recommended MR imaging protocol for diagnosing ON among patients presenting with acute loss of vision, we suggest performing both WBCE-3D T1WI and OCE-2D T1WI in patients with a suspected diagnosis or during follow-up of ON.
Our study has several limitations: First, this is a retrospective study from a single center. Second, readers could not be blinded to the type of sequences they were reading because of their easily recognizable visual features, possibly leading to a certain bias. The randomized pattern of reading we used avoided radiologists’ reading images obtained from the same patient at the same time. Third, our analysis was based only on WBCE-3D T1WI and OCE-2D T1WI. Readers did not have access to other sequences such as coronal T2WI and 3D FLAIR imaging, which have excellent performance for detecting ON, or DWI, proving useful to detect anterior ischemic optic neuropathy.17,18 Our routine MR imaging protocol does not include axial contrast-enhanced 2D T1 fat-suppressed sequences, performed in many centers to assess the optic nerve. This practice may have reduced the diagnostic performance of 2D compared with 3D, considering the ability of readers to reformat the 3D sequence in all planes, not just the coronal plane. Fourth, all our images were acquired exclusively on 3T MR imaging scanners, which may not be representative of the most common MR imaging equipment worldwide and may prevent the generalizability of our results, but this equipment has proved to be more sensitive in detecting optic nerve enhancement compared with 1.5T MR imaging.19 Moreover, the WBCE-3D T1WI we used had a relatively high resolution and optimal fat suppression, which might not be achievable in all centers.
Finally, WBCE-3D T1WI and OCE-2D T1WI sequences were always performed in the same order, with different contrast-enhanced delays, possibly affecting the enhancement of the optic nerve, because the sensitivity of contrast-enhanced images increases with the delay after administration of a gadolinium-based contrast agent.20,21
CONCLUSIONS
Our study showed that there was a strong concordance between contrast-enhanced 3D (WBCE-3D) TSE T1WI and OCE-2D coronal T1WI when detecting enhancement of the optic nerve in patients with acute loss of visual acuity with no obvious ophthalmologic cause. WBCE-3D T1WI showed fewer severe artifacts compared with OCE-2D T1WI and could detect optic disc enhancement. WBCE-3D T1WI demonstrated higher sensitivity and specificity in diagnosing ON, particularly in cases involving the canalicular segments.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received March 19, 2023.
- Accepted after revision February 7, 2024.
- © 2024 by American Journal of Neuroradiology