Abstract
BACKGROUND AND PURPOSE: While the adverse neurodevelopmental effects of prenatal opioid exposure on infants and children in the United States are well described, the underlying causative mechanisms have yet to be fully understood. This study aims to compare quantitative volumetric and surface-based features of the fetal brain between opioid-exposed fetuses and unexposed controls by using advanced MR imaging processing techniques.
MATERIALS AND METHODS: This is a multi-institutional IRB-approved study in which pregnant women with and without opioid use during the current pregnancy were prospectively recruited to undergo fetal MR imaging. A total of 14 opioid-exposed (31.4 ± 2.3 weeks of gestation) and 15 unexposed (31.4 ± 2.4 weeks) fetuses were included. Whole brain volume, cortical plate volume, surface area, sulcal depth, mean curvature, and gyrification index were computed as quantitative features by using our fetal brain MR imaging processing pipeline.
RESULTS: After correcting for gestational age, fetal sex, maternal education, polysubstance use, high blood pressure, and MR imaging acquisition site, all of the global morphologic features were significantly lower in the opioid-exposed fetuses compared with the unexposed fetuses, including brain volume, cortical volume, cortical surface area, sulcal depth, cortical mean curvature, and gyrification index. In regional analysis, the opioid-exposed fetuses showed significantly decreased surface area and sulcal depth in the bilateral Sylvian fissures, central sulci, parieto-occipital fissures, temporal cortices, and frontal cortices.
CONCLUSIONS: In this small cohort, prenatal opioid exposure was associated with altered fetal brain development in the third trimester. This adds to the growing body of literature demonstrating that prenatal opioid exposure affects the developing brain.
ABBREVIATIONS:
- CP
- cortical plate
- FDR
- false discovery rate
- GA
- gestational age
- GI
- gyrification index
- OE
- opioid-exposed
- UE
- unexposed
Prenatal opioid exposure is a known public health crisis in the United States today. The use of opioids during pregnancy is very common and is likely underreported, resulting in substantial associated morbidity and hospital costs.1⇓⇓⇓⇓-6 Opioid-exposed infants have lower birth weights, smaller head circumferences, and neurobehavioral differences in the first weeks after birth, resulting in longer hospital stays compared with unexposed infants.7⇓⇓-10 Long-term studies in children with prenatal opioid exposure reveal lower cognitive scores and poorer school performance in childhood and adolescence.11,12 Despite the scope of this problem, the underlying causative biology of these poor outcomes has yet to be adequately explored. Brain MR imaging studies of opioid-exposed infants and children have revealed smaller regional brain volumes in multiple areas despite no difference in overall brain volume, increased white matter injury, and altered functional networks on resting-state functional connectivity MR imaging compared with unexposed controls.13⇓-15 There are descriptions of potential underlying causes, including alterations in cerebral blood flow, neurogenesis, neuronal differentiation, and neuronal activity as a result of the opioid exposure through the placenta in utero.16 However, postnatal brain imaging, even in the first few weeks after birth, is likely affected by other factors, such as neonatal treatment for opioid withdrawal and breastfeeding. We recently examined fetal brain MRIs in opioid-exposed (OE) and nonexposed fetuses by means of manually measured 2D biometrics that are used in routine clinical practice, which demonstrated multiple smaller brain measurements in the opioid-exposed fetuses compatible with smaller brains.17 More detailed and precise information regarding differences in brain volume and cortical folding between OE and unexposed (UE) fetuses can be detected with advanced fetal MR image processing techniques to extract morphologic features of the brain such as tissue volume, surface area, sulcal depth, mean curvature, and gyrification index (GI). These metrics have the potential to provide a better understanding of the underlying causative mechanisms.
This study aims to compare morphologic features of the fetal brain between opioid-exposed fetuses and unexposed controls by using advanced processing MR imaging techniques.
MATERIALS AND METHODS
Study Design and Patients
This is a prospective multi-institutional study that was HIPAA-compliant and approved by the Institutional Review Board at each institution. Participants were recruited from 3 US academic medical centers: Cincinnati Children’s Hospital Medical Center, Arkansas Children’s Hospital, and the University of North Carolina at Chapel Hill, from July 1, 2020 through December 31, 2021. Patients in the third trimester of pregnancy were recruited to undergo a fetal MR imaging examination for investigational purposes. We recruited women with opioid use during pregnancy from clinics treating women with substance use disorders at each site. Control subjects were recruited from the general public. Substance use, or the lack thereof, was confirmed by questionnaires at the time of the study visit and/or review of maternal charts. Inclusion criteria included age of at least 18 years, singleton pregnancy, and gestational age (GA) of at least 26 weeks. Exclusion criteria assessed by phone interview by a study coordinator included: inability to supply the name of at least 1 additional person to contact in the event that the participant was unable to be reached, fetal abnormality identified on prenatal sonography or known fetal genetic disorder, nonviable fetus, contraindication to MR imaging, and inability to enter the magnet bore due to body habitus. Written informed consent was obtained from all study participants.
Study patients underwent in person detailed questioning at the time of examination by a research coordinator. Substantial opioid use was defined as daily reported opioid use during most of the pregnancy to date, with most enrolled patients on a daily opioid-use disorder maintenance medication, such as buprenorphine or methadone. Polysubstance use was defined as the mother reporting other illicit drugs and/or nicotine during the current pregnancy. Household income was recorded on a 10-point scale based on reported annual income. Maternal education was categorized for each patient as less than 10th grade education level, high school diploma, college enrollment, college degree, and graduate degree. High blood pressure was documented in those that reported pregnancy-related high blood pressure when queried.
MR Imaging Acquisition
Fetal MR imaging examinations were performed by using a 3T Ingenia scanner (Philips Healthcare) at Cincinnati Children’s Hospital, and by using a 3T Prisma scanner (Siemens) at Arkansas Children’s Hospital and University of North Carolina at Chapel Hill. All 3 sites used a phased-array abdominal imaging coil. Patients were not sedated during the examinations and were positioned in the left-side-down decubitus position, unless reporting feeling more comfortable in the supine position. Examinations included T2-weighted single-shot fast/turbo spin-echo sequences (Siemens: TR =1600 ms, TE =101 ms; Philips: TR =5720 ms, TE =101 ms) of the fetal brain in the axial, sagittal, and coronal planes with 2-mm interleaved contiguous slices. Each plane of acquisition was performed at minimum of 1 time each and was repeated as needed to the radiologist’s satisfaction or as allotted study time allowed. Acquisition parameters were standardized across the 3 participating sites. All images were sent to Boston Children’s Hospital for analysis.
MR Imaging Processing and Reconstruction of Cortical Plate Surface
For extracting inner cortical surfaces from MR imaging, we used our pipeline for fetal brain MR imaging processing.18,19 Fetal brains were extracted from raw MR imaging stacks and then intensity inhomogeneity of the brain regions was corrected by N4 bias field correction.20 A motion-corrected 3D volume with 0.75 mm isotropic resolution was created by combining bias field corrected MR imaging stacks by using a section to volume registration technique.21 On the motion-corrected volume, the cortical plate (CP) was segmented by using our deep learning-based approach.22 Using the marching-cube algorithm in CIVET-2.1.0 software package (https://www.bic.mni.mcgill.ca/ServicesSoftware/CIVET), we extracted the inner CP surfaces.23 Initial meshes were tessellated by fitting the boundary between the CP and its inner part, and then resampled to the standard format surfaces containing 81,920 triangles and 40,962 vertices.24 To eliminate small geometrical noise, we applied the Taubin smoothing approach to the inner CP surfaces.25 The smoothed surfaces were registered to a template surface by using a 2D sphere-to-sphere warping method that enables vertex-wise analysis.26 By searching optimal correspondences of vertices by using folding similarity between 2 surfaces, the nonrigid warping method finds vertex correspondence between individual and template surfaces. In this study, a 29 GA template surface was selected as a registration target that was created from similarly reconstructed T2 MR imaging volumes in a different 2D fetal cohort.27
Brain Volume and Surface Measures
Traditional whole brain measures, such as whole brain volume, CP volume, surface area, sulcal depth, absolute mean curvature, and GI, were calculated to examine the difference between 2 groups. Whole brain volume and hemispheric CP volumes were obtained from the segmentation. Surface area was computed by a summation of Voronoi region area at each vertex of the surfaces.28 For sulcal depth, we used the adaptive distance transform that measures the distance of the shortest path from the surface to its convex hull following sulcal geometry.29 Mean curvature was defined as the angular deviation from a vertex patch.28 The sign of mean curvature indicates inwardly folded (negative) or outwardly folded (positive) regions. The absolute mean curvature is taken to ensure the complexity of the brain. To eliminate their noise, we applied spatial smoothing with 10 mm full width at half maximum Gaussian kernel to surface area, sulcal depth, and absolute mean curvature on the surface. As a global feature representing the magnitude of cortical convolution, GI was calculated by the area ratio between the surface and the convex hull.30
Statistical Analysis
To assess group differences in each measure, we employed a linear regression model. In the model, each morphologic feature (whole brain volume, CP volume, surface area, sulcal depth, mean curvature, and GI) was used as a dependent variable while controlling for GA, fetal sex, maternal education, polysubstance use, high blood pressure, and MR imaging acquisition site. For global analysis, we obtained summation of surface area, average sulcal depth, and absolute mean curvature across the vertices. We also employed the Cohen d statistic to assess the difference in measures between 2 groups. Surface area and sulcal depth were independently analyzed as regional analysis, and false discovery rate (FDR) q values were obtained to correct multiple comparisons problems. We set the significance level of FDR q value to 0.05.
RESULTS
Description of Patient Sample
A total of 63 patients completed the MR imaging: 34 from Cincinnati Children’s Hospital Medical Center (OE/UE: 16/18), 16 from University of North Carolina (8/8), and 13 from University of Arkansas for Medical Sciences (4/9). Of these scans, 34 patients were excluded due poor image quality typically related to motion artifact and low SNR that results in poor reconstruction quality. The remaining 29 patients were successfully reconstructed to high-quality 3D volume and included in this analysis: 14 opioid-exposed fetuses (GA: 31.4 ± 2.3 weeks) and 15 unexposed control fetuses (GA: 31.4 ± 2.4 weeks). These and other demographic data are summarized in Table 1.
Description of cohort
Volumetric and Morphologic Measures
All the morphologic measures were significantly lower in opioid-exposed fetuses compared with unexposed control fetuses (Table 2): whole brain volume: Cohen d effect size (P value) = 0.3555 (P < .001), CP volume: 0.2818 (P < .001), cortical surface area: 0.4373 (P < .001), sulcal depth: 0.4739 (P < .001), cortical mean curvature: 0.3120 (P < .001), and GI: 0.4801 (P < .001).
Statistical comparisons of whole brain volume and surface measures between OE and UE control fetuses
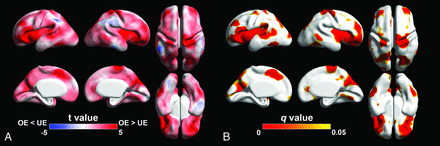
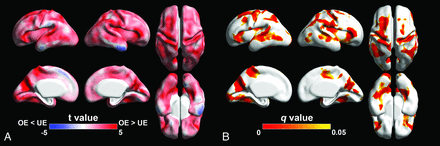
The results of the regional analysis of surface area and sulcal depth between the groups were examined. Opioid-exposed fetuses showed significantly smaller surface area (FDR q < 0.05) compared with unexposed controls in multiple cortical regions, most pronounced in the bilateral Sylvian fissures, central sulci, parieto-occipital fissures, temporal cortices, and prefrontal cortices (Fig 1). Significantly lower sulcal depth in opioid-exposed fetuses was also found in the bilateral Sylvian fissures, central sulci, parieto-occipital fissures, superior frontal sulci, and temporal sulci (Fig 2).
Statistical results of the regional analysis of surface area between OE and UE fetuses. A, Statistical t-map. Red indicates that surface area in OE is smaller than UE fetuses. Blue indicates that surface area in OE is larger than UE fetuses. B, FDR q-map. Red-yellow indicates regions showing statistically different surface area (q < 0.05).
Statistical results of the regional analysis of sulcal depth between OE and UE. A, Statistical t-map. Red indicates that sulcal depth in OE is shallower than UE fetuses. Blue surface area in OE is deeper than UE fetuses. B, FDR q-map. Red-yellow indicates regions showing statistically different sulcal depth (q < 0.05).
DISCUSSION
This prospective, multi-institutional study is one of the first reports of volumetric and morphometric brain parameters on MR imaging of opioid-exposed fetuses. We demonstrate statistically significant lower total brain volumes, CP volumes, and surface areas in the brains of OE fetuses compared with UE controls, even after correcting for potentially confounding variables. We also demonstrate statistically significant decreased mean sulcal depth, cortical mean curvature, and GI in OE fetuses compared with UE controls. Finally, we observed regional predominance of differences in surface area and sulcal depth between the 2 groups in multiple areas of the developing cortex, most notably involving the bilateral perisylvian cortices, perirolandic cortices, and cortices along the parieto-occipital fissures.
Decreased head circumference in infants with prenatal opioid exposure has been well described in the literature, presumed to be a secondary finding of decreased brain volumes.8,31⇓-33 There is also evidence of decreased regional brain volumes on MR imaging in infants with prenatal opioid exposure, particularly involving the deep gray structures.13,34 Studies extending into the prenatal period are more sparse. In a previous study including this group of patients, we compared multiple 2D measurements of the fetal brains in opioid-exposed fetuses with unexposed controls and found multiple parameters that were lower in the opioid-exposed group.17 The current study expands on these previous findings by employing advanced MR processing techniques, indicating that 2D measurements used in clinical practice are indeed concordant with volumetric analyses. In addition, the current study adds morphometric data to include lower CP volume and surface area in opioid-exposed fetuses, which has not been previously described. Decreased brain size is a manifestation of a decreased number of neurons, which is presumed to be related at least in part to an abnormality of neuronal proliferation.35 There is literature demonstrating evidence of impaired neurogenesis, proinflammatory changes, and increased apoptosis in rat models with prenatal opioid exposure, which would in part explain our findings.36⇓⇓-39
Our study also adds information on prenatal gyration of the opioid-exposed fetus, which appears to be delayed compared with unexposed fetuses. The reason for this is less well understood; however, it is believed that the formation of gyri and sulci in the brain is in part a response to the dramatic increase in brain size that occurs during development, the folding of the brain allowing it to fit within the confines of a cranial vault that is small enough to accommodate the birthing process.40 As such, the impairment in neuronal proliferation may be at least part of the cause of the altered cerebral organization. There is also literature describing evidence of chronic opioid use causing desensitization of opioid receptors in the placenta, which play key roles in modulating neuronal migration and differentiation in the fetus, which may also in part explain differences in gyration.16 We also observed regional differences in surface area and sulcal depth in multiple functionally critical areas of the developing brain with a predominance in the primary fissures (Sylvian and parieto-occipital) and sulci (central, temporal, and superior frontal), which are some of the first areas of cortical folding we visualize in clinical practice, indicating that structural effects of opioid exposure have already begun early in the second trimester and likely have ongoing or downstream effects on the remaining steps of brain development.41 The cerebral white matter microstructure also appears to be altered in infants and children with prenatal opioid exposure, as illustrated in studies examining differences in DTI parameters.42⇓⇓-45 Because cortical folding is driven in part by regional specialization and developing corticocortical connections, altered folding may be related to differences in DTI metrics observed in neonates. There is even early evidence to suggest that these white matter structural alterations begin in utero when examining fetal DTI parameters.46 Finally, there are also a number of papers describing alterations in functional connectivity in opioid-exposed infants on resting-state fMRI compared with unexposed infants, which may also be guiding cortical organization.47⇓⇓-50
As a result of its multi-institutional nature, we contribute one of the largest cohorts of opioid-exposed fetuses to the existing MR imaging literature. This allowed us to control for multiple potentially confounding variables, including nicotine exposure, fetal sex, high blood pressure, and maternal education. Smaller brain volumes have been documented in fetuses with prenatal nicotine exposure, making it an important confounding variable to control for in this population.51,52 Differences in brain volumes and growth patterns have also been described on fetal MR imaging between male and female fetuses, making this another important confounding variable to account for.53,54 Although the existing literature is limited, we also chose to control for patients who reported high blood pressure during pregnancy because there is evidence of neurologic alterations in children born to pre-eclamptic and hypertensive mothers.55 Finally, we chose to control for maternal education because this has been linked to differences in fetal brain volumes and is associated with other factors affecting fetal brain development including maternal occupation, household income, and material resources.56
Our study has a number of limitations. One of the major limitations of this study is that, despite its multi-institutional nature, the overall sample size is relatively small. Another limitation commonly encountered in fetal MR imaging are imaging artifacts, particularly those related to fetal motion. Field inhomogeneity, aliasing, and dielectric effect are other artifacts that compromise image quality. Despite implementation of advanced motion correction techniques, more than one-half of the patients examined had to be excluded for excessive fetal motion in this study. Finally, one of the main limitations of this study relates to the factors potentially associated with both opioid exposure and brain development. Although we controlled for nicotine and polysubstance exposure in our analysis, there may have been other unmeasured factors associated with both opioid exposure and brain development, such as maternal nutrition, stress, and other environmental factors. This highlights the need for future studies in larger patient populations to better isolate these variables and better understand these early findings.
CONCLUSIONS
This study demonstrates decreased volumes and gyration of the developing fetal brain in fetuses with prenatal opioid exposure compared with unexposed controls by using advanced fetal MR imaging processing techniques. These findings may be related at least in part to opioids causing impaired neurogenesis in the developing brain; however, given the complexity of human brain development, there are likely multiple underlying mechanisms affected. Fetal MR imaging may prove to be a valuable tool in identifying the effects of opioid exposure in utero and potentially disentangling the impact of postnatal confounding factors. Further studies in larger populations are critical to better understanding these findings.
Footnotes
H.J. Yun and U.D. Nagaraj contributed equally to this work.
This work was supported by Schubert Research Clinic Clinical Research Feasibility Fund, National Institutes of Health Planning Grant Program (Cincinnati Children’s Hospital Medical Center R34-DA050268, ACRI R34-DA050261, University of North Carolina R34-DA050262), R01NS114087, R01HD100009, and R01EB032708.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
REFERENCES
- Received September 14, 2023.
- Accepted after revision November 7, 2023.
- © 2024 by American Journal of Neuroradiology