Abstract
BACKGROUND AND PURPOSE: Nonspecific, localized thalamic signal abnormalities of uncertain significance are occasionally found on pediatric brain MR imaging. The goal of this study is to describe the MR imaging appearance and natural history of these lesions in children and young adults.
MATERIALS AND METHODS: This retrospective study evaluated clinically acquired brain MR imaging examinations obtained from February 1995 to March 2022 at a large, tertiary care pediatric hospital. Examinations with non–mass-like and nonenhancing thalamic lesions were identified based on term search of MR imaging reports. A total of 221 patients formed the initial group for imaging assessment. Additional exclusions during imaging review resulted in 171 patients. Imaging appearance and size changes were assessed at baseline and at follow-up examinations.
RESULTS: A total of 171 patients (102 male) at a median age of 11 years (range: 1–23 years), 568 MR imaging examinations, and 180 thalamic lesions were included. Median time from baseline to the last follow-up MR imaging was 542 days (range: 46–5730 days). No lesion enhanced at any time point. On imaging follow-up, 11% of lesions (18/161) became smaller, 10% (16/161) resolved, 73% (118/161) remained stable, and 6% (9/161) increased in size at some point during evaluation. Median time interval from baseline to enlargement was 430 days (range: 136–1074 days).
CONCLUSIONS: Most incidental, non–mass-like thalamic signal abnormalities were stable, decreased in size, or resolved on follow-up imaging and are likely of no clinical significance. Surveillance strategies with longer follow-up intervals may be adequate in the management of such findings.
ABBREVIATIONS:
- AP
- anteroposterior
- EMR
- electronic medical record
- GRE
- gradient recalled-echo
- PD
- proton density
- TR
- transverse
Increasing utilization and technical advances in MR imaging have led to the frequent identification of incidental findings (unexpected imaging findings likely unrelated to clinical presentation) on pediatric brain MR imaging examinations performed for both clinical and research purposes.1⇓⇓⇓-5 In our pediatric neuroradiology practice, we often identify nonspecific lesions within the thalami on brain examinations obtained for a wide variety of indications. While some of these incidental findings may have immediate clinical implications, most are of uncertain significance and may produce confusion among clinicians and concern among patients and their families.6 In addition, subsequent evaluations of these findings may lead to cascades of additional imaging and testing and increased health care expenses. Outcomes-based research is an important pillar in the development of management recommendations for incidental findings.7,8 While prior studies have assessed incidentally identified, mass-like, potentially neoplastic signal abnormalities in the thalamus in children,6,9 we are unaware of any investigation assessing the imaging appearance, distribution, and natural history of non–mass-like, nonspecific, areas of signal abnormality in the thalamus in the pediatric population. Based upon our clinical experience, we hypothesized that these signal abnormalities rarely change on follow-up imaging and rarely have definite clinical significance. The purpose of our study was to assess the imaging appearance of these signal abnormalities and their natural history on follow-up examinations (when available), describe basic clinical associations, and define reasonable MR imaging follow-up guidelines.
MATERIALS AND METHODS
This retrospective study was approved by the Institutional Review Board at Cincinnati Children’s Hospital Medical Center. All research activities were Health Insurance Portability and Accountability Act compliant, and the need for patient informed consent was waived.
Sample Selection
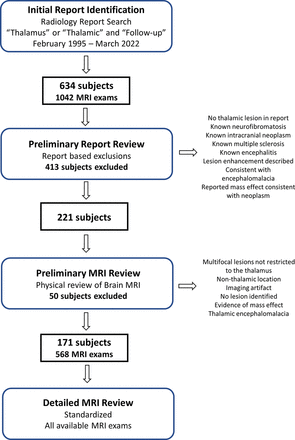
Patients were identified by performing a radiology report search (Illuminate Insight, Softek) of brain MR imaging examinations performed between February 1995 and March 2022 (Fig 1). Reports were selected that included both the terms “thalamic” and/or “thalamus” and “follow-up” in the radiology report. A total of 634 potential patients (1042 MR imaging examinations) were identified on initial report search. A preliminary radiology report review was performed by 2 board-certified pediatric neuroradiologists (both with >20 years clinical experience; J.L.L., M.M.C.) with the following exclusions: No thalamic lesion described in the identified report; known history of neurofibromatosis, intracranial neoplasm, multiple sclerosis, or encephalitis at the time of reporting; and reported contrast enhancement on the baseline examination, thalamic encephalomalacia, or thalamic lesions with mass effect consistent with neoplasm. A total of 413 patients were excluded by this preliminary report review. Then, a preliminary imaging review of the report-identified MR imaging examinations in the remaining 221 patients was performed by the same 2 board-certified pediatric neuroradiologists. Additional exclusions during preliminary imaging review included the following: multifocal lesions not restricted to the thalamus, nonthalamic location of the reported signal abnormality, imaging artifact responsible for the abnormality, no lesion identified on imaging review, evidence of mass effect (including distortion of internal thalamic structures, expansion of the thalamus, deformity of adjacent CSF spaces indicating mass effect), or imaging findings consistent with thalamic encephalomalacia. An additional 50 patients were excluded after preliminary MR imaging review for a final cohort of 171 patients with nonspecific, non–mass-like thalamic signal abnormalities. All previous and follow-up brain MR imaging examinations available in our institutional PACS were identified in this cohort for a total of 568 brain MR imaging examinations.
Flow chart of study participant selection process. Conditions on right denote exclusion criteria.
Extraction of Clinical Data
Clinical and demographic data were acquired from the electronic medical record (EMR) (Epic Systems) and imaging reports by a research fellow (V.d.P.V.A.). The clinical indication for the examination on the radiology report was extracted. If a patient had multiple follow-ups, the clinical indication for the MR imaging was extracted from the first examination where the thalamic abnormality was mentioned. The EMR was systematically searched utilizing the most recent clinical notes available and the continually updated problem list and extracted for the patients with known medical conditions. The results were summarized into pertinent categories based on the timing of the initial MR imaging where the thalamic lesion was identified and based on a combination of radiology report clinical indications and the EMR search.
Standardized MR Imaging Review of Thalamic Abnormalities
Detailed imaging review of brain MR imaging studies was performed by the same 2 board-certified neuroradiologists (J.L.L., M.M.C.). An initial 10 training studies were evaluated by both reviewers together to define and agree upon measurement method and lesion characteristic assessment. Subsequently, all examinations were independently evaluated by each examiner. One reviewer (J.L.L.) evaluated 65% of examinations (368/568), whereas the other (M.M.C.) evaluated 35% (200/568) of the examinations. For patients with multiple MR imaging examinations, the first occurrence of a thalamic lesion was considered the baseline MR imaging examination. Image assessment and lesion measurement were performed utilizing the clinical PACS system at our institution. Imaging reviewers were not blinded to the clinical MR imaging report. The reviewers assessed thalamic lesion characteristics and their longitudinal change on each examination utilizing a standardized electronic form. Lesion location was defined by nuclear group (anterior, posterior, lateral, and medial) utilizing a standardized template reference10 and brain hemisphere lateralization (left and right). Lesion signal intensity was visually characterized for each sequence compared with the ipsilateral caudate nucleus (hypointense, isointense, hyperintense) or nonvisible/indistinguishable from normal thalamus for each available sequence, including T1-weighted, T2-weighted, proton attenuation (PD), T2-weighted FLAIR, DWI, ADC maps, and gradient recalled-echo or susceptibility weighted imaging (GRE/SWI). Signal was described as heterogeneous or homogeneous, and margins as ill-defined or well-defined. Well-defined margins were defined as sharply circumscribed transitions around the entire circumference of the lesion. The MR imaging sequence allowing best visualization of the lesion was used for measurement (typically, the axial T2-weighted or T2 FLAIR sequence). Anteroposterior (AP) and transverse (TR) linear measurements were performed and recorded for each lesion. Any change from prior examinations were denoted. Assessment and documentation of any MR imaging studies performed before identification of the thalamic abnormality were also performed. Patient sex and age were obtained from imaging reports at the time of first MR imaging (if more than 1 examination was present).
MR Imaging Procedures
A total of 373 (66%, 373/568) examinations were performed on 1.5T MR scanners, and 195 (34%, 195/568) were performed on 3T MR scanners. Given the retrospective nature and time frame of the study, MR imaging protocols and scanner manufacturer varied, but each examination included at least T1-weighted and T2-weighted pulse sequences, at a maximum of 6 mm section thickness (range: 1–6 mm, median: 4 mm). Additional pulse sequences, available on a case-by-case basis included the following: PD, T2 FLAIR, DWI, ADC maps, and GRE/SWI. A total of 343 (60%, 343/568) examinations were performed on GE Healthcare scanners, 171 (30%, 171/568) on Philips Healthcare scanners, and 52 (10%, 52/568) on Siemens scanners. A total of 408 (70.5%, 408/578) studies included the administration of gadolinium-based contrast agents during initial identification and/or follow-up of the thalamic signal abnormalities.
Statistical Analysis
A descriptive analysis of demographic and clinical data was performed to summarize sample and imaging characteristics. All statistical analyses were performed with Excel Version 2209 Build 16.0 (Microsoft). Categoric variables were presented as percentages. For enlarging lesions, AP and TR dimensional cross product at each imaging time point was plotted overtime to graphically demonstrate growth characteristics.
RESULTS
Study Sample Characteristics
A total of 171 patients (102 male, 69 female) with 568 MR imaging examinations and 180 thalamic lesions were included in this study. Median age at baseline MR imaging was 11 years (range: 1–23 years). The 2 most common indications for the MR imaging examination were headaches (43%, 73/171) and seizures/epilepsy (23%, 40/171) (Table 1). No patient had a clinical diagnosis of neurofibromatosis or multiple sclerosis at the time of the initial examination, or subsequently, by review of the EMR up until December 1, 2022.
Clinical scenario for baseline MR imaging examinations (n = 171)
Thalamic Lesion Characteristics at Baseline MR Imaging
Among all lesions, 91 (50.5%, 91/180) were located in the right thalamus and 89 (49.5%, 89/180) in the left thalamus. A total of 109 lesions (61%, 109/180) were identified in the posterior, 51 (28%, 51/180) in the lateral, 17 (10%, 17/180) in the medial, and 3 (1%, 3/180) in the anterior thalamic nuclear regions. Imaging examples of typical lesions are provided in Figure 2. The median size of the lesions was as follows: AP, 4 mm (range: 2–21 mm); TR, 5 mm (range: 1–18 mm). For border definition, 128 lesions (71%, 128/180) were considered ill-defined, whereas 52 lesions (29%, 52/180) were classified as well-defined. For internal architecture, most lesions (97%, 174/180) were deemed homogeneous and a minority (3%, 6/180) were categorized as heterogeneous. No lesion exhibited contrast enhancement on any follow-up examinations. Table 2 provides the signal characteristics of the lesions based on different MR imaging sequences. Lesions were best seen on T2 or T2-FLAIR sequences and were most commonly hyperintense on T2 and T2 FLAIR (77.3%, 79.9%, respectively) and isointense to hypointense on T1-weighted images (42.5%) compared with the caudate. While most lesions were not identified on DWI/DTI, some (11.1%) exhibited increased diffusivity relative to the thalamus and caudate. None demonstrated relative diffusion restriction on ADC maps.
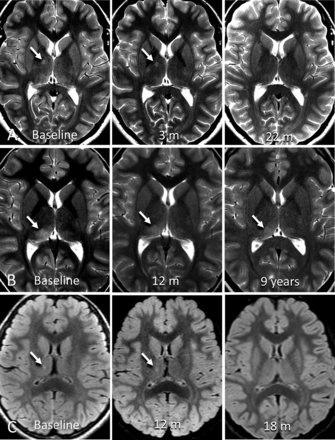
Example cases of incidental thalamic lesions identified in this study. A, Axial T2-weighted images of a 13-year-old adolescent girl with headache show a focal thalamic lesion (arrow) within the anterior lateral right thalamus on baseline MR imaging. The lesion was stable on the 3-month follow-up and resolved at 22-month follow-up. B, Axial T2-weighted images of a 9-year-old girl with headache show ill-defined thalamic signal (arrow) in the posterior right thalamus on baseline MR imaging. At 1-year follow-up, the lesion was slightly less defined and smaller. At 9-year follow-up, the lesion was smaller and less defined. This patient had 12 follow-up scans for this lesion over a 9-year period. C, Axial T2 FLAIR images of a 5-year-old girl after a single seizure episode. Baseline MR imaging shows a small focal signal abnormality in the right thalamus (arrow). This lesion slightly enlarged at 1-year follow-up and resolved at 18-month follow-up imaging.
Thalamic lesion signal characteristics on baseline MR imaging (n = 180 lesions)
Longitudinal Analysis on Follow-up MR Imaging
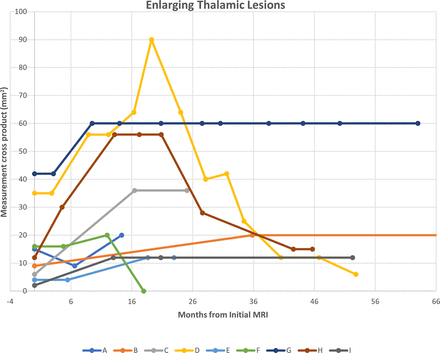
A total of 152 (89%, 152/171) patients had follow-up examinations with a median of 2 follow-up examinations (range: 1–12 examinations). A total of 87 subjects (51% of total cohort) had 1-year or greater follow-up: 1–2 years: 21; 2–3 years: 21; 3–4 years: 19; 4–5 years: 13; >5 years: 13. A 3-month follow-up interval was most commonly recommended in baseline scan reports (44%, 76/171), ranging from 1 to 12 months (Table 3). Median time from baseline to the last follow-up MR imaging was 542 days (range: 46–5730 days). A total of 161 lesions (89%, 161/180) were followed up longitudinally. Of those, 18 (11%, 18/161) became smaller, 16 (10%, 16/161) resolved, 118 (73%, 118/161) remained stable, and 9 (6%, 9/161) were larger at any follow-up point (of these: 1 had no further follow-up, 6 were subsequently stable or smaller, and 1 resolved) (Fig 3). One enlarging lesion (maximum dimension of 10 × 8 mm) was treated with radiation therapy for presumed thalamic glioma (without histologic confirmation) with a subsequent decrease in size (Fig 3C). Among the enlarging lesions, the median interval from baseline to initial identified enlargement was 430 days (range: 136–1074 days). Four lesions enlarged within 1 year, and 4 within 2 years. Graphical representation of lesion growth is provided in Figure 4. No lesions were biopsied. No other subject, by review of the EMR up until December 1, 2022, had clinical diagnosis of intracranial neoplasm.
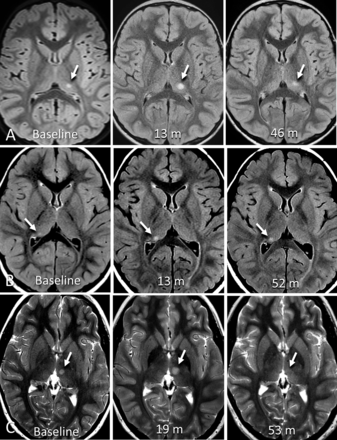
Example cases of enlarging incidental thalamic lesions identified in this study. A, Axial T2 FLAIR images of a 6-year-old boy with headache show a focal thalamic lesion (arrow) within the posterior left thalamus on baseline MR imaging. At 13 month follow-up, the lesion was enlarged. At 46-month follow-up, the lesion was more ill-defined and slightly decreased posteriorly. B, Axial T2 FLAIR images part of routine follow-up of a 4-year-old boy with history of right cerebellar complex developmental venous anomaly. Baseline MR imaging shows a small focus of increased signal in the posterior right thalamus, enlarged at 13-month follow-up, then stable 52 months after baseline MRI. C, Axial T2-weighted images of an 8-year-old girl with history of head trauma and headache showing a focal lesion within the left medial thalamus (7 × 5 mm). There was slow interval enlargement over 5 MR imaging studies for 19 months, at which point the lesion was classified as presumed low-grade glioma, and was treated with a total of 50.4 Gy fractionated radiation therapy over 8 weeks. Following therapy, there was a decrease in the site over subsequent 34 months.
Growth trajectories of 9 thalamic lesions that enlarged at any time during follow-up MR imaging. Patient D, an 8-year-old girl with history of head trauma and headache, was treated with radiation therapy 19 months after initial lesion identification after growth identified with a subsequent decrease in size. Patient B, a 14-year-old adolescent boy with history of fetal alcohol syndrome and tethered cord, had additional stable follow-up examinations at 138 and 190 months (not shown).
MR imaging follow-up recommendations available on baseline imaging reports (n = 171 patients)a
In subjects with ≥2 years follow-up (excluding the radiation therapy treated subject), no lesion that had been stable or smaller (including those that had previously enlarged and were subsequently stable or smaller) by 2 years continued to enlarge. One subject (Subject B, Fig 3) had an enlargement time point at 2.9 years (1074 days); however, this patient had no interval studies from their initial brain MR imaging. This subject was followed for an additional 4656 days (12.8 years) with long-term stability.
A total of 26 patients had MR imaging examinations performed before the identification of thalamic signal abnormalities. Mean time from the most recent prior study (where no thalamic lesion was identified) to the first MR imaging examination demonstrating a thalamic lesion was 1882 days (range: 148–5437 days). Six subjects had 2 thalamic lesions at initial diagnosis, all stable on subsequent follow-up (6 months to 2 years). Three subjects developed a new thalamic lesion on follow-up imaging for an existing thalamic lesion. One did not have follow-up imaging after the new lesion was identified (6 years after the initial examination); thus, growth potential cannot be documented. Of those that had follow-up, 1 new lesion was stable at subsequent 2-year follow-up, and 1 new lesion was smaller at subsequent 4-month follow-up. No subjects with resolved thalamic lesions had subsequent follow-up brain imaging studies.
Most thalamic lesions, even those described as having ill-defined margins, were well delineated relative to the remainder of the thalamus. During data analysis, a subgroup of thalamic lesions, all within the pulvinar of the posterior thalamus (n = 21), were identified with very ill-defined margins that made differentiation of lesion borders challenging (Fig 2B). In these patients, measurements were often difficult. There was no thalamic expansion or mass effect (by definition). Of the 20 lesions in this subgroup with follow-up imaging, 19 were stable and 1 resolved.
No thalamic imaging finding specifically correlated with the clinical scenario at diagnosis. After the baseline examination, no subject developed a diagnosis of multiple sclerosis, neurofibromatosis, metabolic condition, or other condition that could be specifically related to the thalamic abnormality.
DISCUSSION
Pediatric brain MR imaging studies conducted for clinical and research purposes often harbor incidental imaging findings.1 The reported rates of such findings vary from 10% to 26% of examinations.3,4,6,11 Our retrospective study included 171 children, adolescents, and young adults who underwent brain MR imaging for various indications, the most common being headache, seizures, and epilepsy. We found that incidentally identified, non–mass-like, nonspecific, thalamic lesions were mostly (94%) stable, smaller, or resolved on follow-up imaging. Among the lesions that enlarged (6%), most were subsequently stable, smaller, or resolved. One enlarging lesion was treated with radiation therapy as a presumed thalamic neoplasm with subsequent decrease in size. The thalamic lesions were most frequently located in the posterior and lateral thalamic groups and were ill-defined and exhibited no diffusion restriction. No lesions were biopsied or underwent resection. No lesion with follow-up imaging that included contrast administration subsequently enhanced. None of the thalamic imaging findings was definitely correlated with the examination clinical scenario. Together, our study suggests that most of these small, nonspecific, non–mass-like thalamic signal abnormalities are of limited clinical significance.
Developing effective imaging follow-up and management strategies for incidentally identified findings on brain MR imaging requires a thorough understanding of their imaging characteristics and outcomes and has been little studied in pediatric patients.1,8,9 Zaazoue et al6 evaluated 144 pediatric patients with incidental brain lesions on MR imaging indeterminate for tumor, including 26 patients with thalamic lesions. In their study, only 3 patients with thalamic lesions showed an increase in lesion size on follow-up imaging, and none required surgical intervention. Like our investigation, those with typical syndromic characteristics, such as NF1, NF2, and tuberous sclerosis, were excluded. However, the authors included only lesions thought to potentially represent neoplasms, and included lesions with mass effect which makes their cohort essentially different from ours. Although their numbers were small, their outcomes were comparable to ours, and they concluded that patients with thalamic lesions are less likely to progress or require surgical intervention than those in other locations.6
Additional research on pediatric MR imaging has focused primarily on thalamic lesions with mass effect or high suspicion for neoplasm.6,9 For instance, Kozyrev et al9 investigated 58 children with space-occupying thalamic lesions incidentally identified on MR imaging, of whom 21 underwent surgery due to clinician suspicion of high-grade tumor, change in lesion characteristic, or growth on follow-up imaging. In their study, contrast-enhancing lesions were included and most operated patients were found to have low-grade or high-grade gliomas on histopathologic analyses. In contrast, we excluded all lesions demonstrating mass effect and/or contrast enhancement. However, because many of their thalamic neoplasms, including those that were both low and high grade, were nonenhancing, enhancement characteristics may not be a key distinguishing factor.
In our study, no histopathology analyses were performed in any of the thalamic lesions, which prevents confirmation of etiology. The differential diagnosis for an imaging lesion in the thalamus is wide ranging.12 Some of the lesions may represent small, indolent, or spontaneously regressing neoplasms. Prior studies have reported on spontaneous resolution of low-grade tumors in pediatric patients.13,14 Transient edema or inflammation may account for the complete resolution of some lesions observed in imaging, particularly in patients with a history of seizures or epilepsy. Investigations involving patients with prolonged status epilepticus have revealed thalamic DWI and FLAIR hyperintensities on the same side as the epileptiform activity,15,16 often ill-defined signal within the pulvinar.17 Thalamic involvement has also been associated with venous vasculitis conditions, such as Behcet disease, or connective tissue disorders, such as Sjogren syndrome.15 These lesions often appear hyperintense in T2 and FLAIR sequences and occasionally exhibit enhancement in gadolinium-enhanced T1-weighted imaging.15 Finally, these lesions may represent isolated dysplastic or hamartomatous changes of the thalamus, such as in cases of NF112,18 (although no subject in our study had this diagnosis).
Our study has several limitations. As this was a retrospective analysis of examinations from a single institution, there is potential for selection bias in our results. Therefore, future prospective and multicenter studies are needed to confirm our findings. The inclusion of images dating back to 1995 means that there were substantial differences in acquisition protocols and techniques between examinations, which may have impacted the identification and characterization of thalamic lesions. In addition, differences in section thickness selection between examinations may have contributed to apparent changes in lesion size as many lesion dimensions were near or below the section thickness of examinations. Without histopathology, the potential etiologies of the lesions remain speculative. Our image reviews were subjective and relied on the expertise of 2 readers who assessed different cases, which may have introduced variability in our results. However, both neuroradiologists reviewed a common training set of 10 subjects to define measurement technique and subjective assessment, trained in an identical fashion at the same institutions, and have been working in the same institution together since 2007, limiting this potential confounding factor. Of 171 subjects, 66 subjects (39%) had imaging follow-up for >2 years. This does limit our assessment for very slow growing tumors and could occur for a number of many reasons including: no report recommendations for continued follow-up, clinician/patient choice, transfer to adult or other pediatric institutions, or loss of medical follow-up. This limits our ability to establish definitive data on very long-term follow-up of these findings and is an unavoidable limitation of a retrospective study. On clinical chart review, none of our subjects had a subsequent diagnosis of tumor or other clearly related clinical condition (other than the subject treated with radiation therapy for presumed glioma without histologic proof, which enlarged at 7 months after identification).
The findings of this study may be used to guide and improve imaging follow-up recommendations for incidental, nonspecific, thalamic lesions. Considering that all enlarging lesions (ie, more concerning for neoplasia) in our cohort did so by 3 years from initial imaging, a less aggressive surveillance strategy may be adequate. Based on imaging reports, the suggested follow-up intervals from our cohort (Table 3) were predominantly ≤6-month intervals, which may have been unnecessarily conservative, given our outcome findings. Additionally, assessing our subset of subjects with longer-term follow-up, no lesion with prior follow-up imaging followed for >2 years demonstrated enlargement. One lesion enlarged at 2.9 years after initial diagnosis (without previous follow-up imaging), and was subsequently stable for 12.8 years. Conclusions, however, are limited by lack of > 1 year imaging follow-up in many subjects. If follow-up imaging is deemed necessary, it may be beneficial to extend the follow-up period to 6–12 months initially, followed by yearly follow-ups thereafter for at least 3 years after diagnosis. By adopting this management approach, we can ensure that patients receive appropriate monitoring while additionally minimizing unnecessary diagnostic testing.
CONCLUSIONS
In our retrospective study of 171 children, adolescents, and young adults who underwent brain MR imaging for various neurologic or screening indications, most incidentally identified, non–mass-like thalamic signal abnormalities were stable, smaller, or resolved on follow-up imaging and likely of minimal to no clinical significance. Of lesions that enlarged with subsequent follow-up imaging, 1 continued to enlarge, concerning for neoplasm. Therefore, less conservative surveillance strategies of such incidental findings with longer follow-up intervals may be adequate to guarantee both optimized and cost-effective care for these patients.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
REFERENCES
- Received September 19, 2023.
- Accepted after revision November 6, 2023.
- © 2024 by American Journal of Neuroradiology