Abstract
BACKGROUND AND PURPOSE: White matter injury in infants born preterm is associated with adverse neurodevelopmental outcomes, depending on the extent and location. White matter injury can be visualized with MR imaging in the initial weeks following preterm birth but is more commonly defined at term-equivalent-age MR imaging. Our aim was to see how white matter injury detection in MR imaging compares between the 2 time points.
MATERIALS AND METHODS: This study compared white matter injury on early brain MR imaging (30–34 weeks’ postmenstrual age) with white matter injury assessment at term-equivalent (37–42 weeks) MR imaging, using 2 previously published and standardized scoring systems, in a cohort of 30 preterm infants born at <33 weeks’ gestational age.
RESULTS: There was a strong association between the systematic assessments of white matter injury at the 2 time points (P = .007) and the global injury severity (P < .001).
CONCLUSIONS: Although the optimal timing to undertake neuroimaging in the preterm infant remains to be determined, both early (30–34 weeks) and term-equivalent MR imaging provide valuable information on white matter injury and the risk of associated sequelae.
ABBREVIATIONS:
- CUS
- cranial ultrasound
- IVH
- intraventricular hemorrhage
- NICU
- neonatal intensive care unit
- PMA
- postmenstrual age
- TEA
- term-equivalent age
- WMI
- white matter injury
White matter injury (WMI) in the preterm infant is common and associated with adverse neurodevelopmental outcomes.1,2 It remains the most prevalent form of brain injury in the vulnerable, developing brain, with the highest risk for those infants born between 23 and 32 weeks’ gestational age.3 While severe cystic WMI is seen in <5% of infants born at <32 weeks’ gestational age, focal necroses appearing as punctate white matter lesions in MR imaging are seen in 15%–25% of infants born at <28 weeks, and up to one-half of them may have diffuse lesions with gliosis, which results in subsequent white matter volume loss.3 Cranial sonography (CUS) is the most widely used and readily available neuroimaging technique in the neonatal intensive care unit (NICU). It is particularly useful and sensitive for the detection of intraventricular hemorrhage and its short-term sequelae such as ventriculomegaly. CUS is also able to detect more severe white matter lesions such as cystic periventricular leukomalacia. However, it has limited sensitivity to detect focal, punctate WMI, or diffuse WMI.4 Term-equivalent age (TEA) (37–42 weeks) MR imaging has been shown to have greater sensitivity than CUS for diffuse WMI and punctate white matter lesions or their sequelae such as white matter volume loss.4⇓-6 Early MR imaging, often performed between 30 and 34 weeks’ postmenstrual age (PMA), appears to be most sensitive for the direct visualization of the extent and nature of WMI.7 These early lesions are subsequently replaced by microglia and astroglia and evolve into cystic spaces or glial tissue, often becoming less conspicuous with time.6,7 WMI may result in white matter volume loss with a secondary dysmaturational impact leading to reductions in deep and cortical gray matter volume.3 These secondary dysmaturational impacts of WMI may be best visualized at TEA.8,9
Systematic MR imaging scoring methods to assess the extent of WMI have been proposed for both early and TEA times.5,7 Although there are similar elements in both scoring systems, early MR imaging interpretation focuses primarily on characterization of the lesions of WMI,7 whereas TEA scores capture WMI sequelae such as cystic lesions, signal abnormalities, delayed myelination, and white matter volume loss.5 There is no current consensus on the best timing and use of MR imaging in the preterm population, but there is growing evidence that neonatal brain MR imaging offers information that most clearly delineates brain injury with implications for rehabilitation.10 Thus, the aim of this study was to examine early and TEA brain MRIs in the same cohort of infants born at <33 weeks’ gestational age using 2 standardized scoring systems and to assess how WMI and overall scores compared between the 2 time points.
MATERIALS AND METHODS
Study Population
Participants of the study were enrolled from Brigham and Women's Hospital, a 66-bed level 3 NICU within a single academic institution in Boston, Massachusetts. From September 2020 to October 2022, we recruited preterm infants born at <33 weeks’ gestational age with no congenital anomaly and no congenital infections. The study was approved by the Mass General Brigham Institutional Review Board, and parents of each participant provided written informed consent.
Brain MR Imaging Acquisition and Scoring
Brain MR imaging was performed at an early time point (30–34 weeks’ PMA), and at TEA (37–42 weeks PMA)5 for all study participants. Infants were imaged after feeding and swaddling, without any sedation. The MR imaging performed at both time points was clearly interpretable with minimal or no artifacts, including those due to motion.
Early MR images were obtained in a 1T in-NICU Embrace MRI system (Aspect). Our team previously described the feasibility of obtaining imaging in hospitalized infants using an in-NICU scanner11 in our setting. MR imaging included anatomic T1- and T2-weighted imaging and diffusion-weighted sequences. The acquisition parameters for the sequences in this system were T1-weighted fast spin-echo (section thickness = 3–4 mm, TR = 600 ms, TE = 12 ms), T1-weighted 3D gradient-echo (TR = 20 ms, TE = 3.5 ms, section thickness = 1 mm), T2-weighted fast spin-echo (TR = 10–13 seconds, TE= 130–150 ms, section thickness = 3–4 mm), and diffusion-weighted TSE imaging (TR = 14 seconds, TE = 125 ms, section thickness = 3–4 mm, diffusion b-value = 1000). The TEA MR images were acquired using a 3T scanner (Siemens). Sequences included T1-weighted sequences (isotropic voxel size = 1 mm3, TR = 2540 ms, TE = 3.4 ms, TI = 1450 ms), T2-weighted (isotropic voxel = 1 mm3, TR = 16 seconds, TE = 136 ms), and diffusion-weighted sequences (isotropic voxel size = 2 mm3, 104 diffusion directions [10, 30, and 64 directions at b=500, 1000, and 2500 s/mm2, respectively], TE = 71 ms, TR = 3.5 seconds).
Because previous studies have reported reassuring developmental outcomes in infants with normal-mild WMI,6 we aimed to assess the consistency of 2 broad categories of normal-mild and moderate-severe WMI (and global brain injury) as assessed by scoring systems applied to early and TEA MRIs. The scoring system elaborated by Miller et al7 was used for categorization of WMI as well as global brain injury on early MR imaging. This score has been shown to have robust interrater reliability along with prognostic utility on follow-up.7 The severity of WMI is classified as normal (no white matter lesions), minimal (≤3 areas of T1 signal abnormality, each measuring <2 mm), moderate (>3 areas of T1 signal abnormality or these areas measuring >2 mm, but <5% of the hemisphere involved), or severe (>5% of the hemisphere involved). To define 5% of a hemisphere, the image in which the echogenicity is most clearly appreciated is selected and the ipsilateral hemisphere is divided into 5 equal pie-like segments. A >5% involvement would entail an echogenicity size of more than one-fourth of a single segment. An overall severity score considers the presence and degree of intraventricular hemorrhage (IVH) and ventriculomegaly in addition to WMI. The global moderate-to-severe category includes moderate-or-severe white matter injury, any ventriculomegaly (defined as a ventricular diameter of >8 mm), or severe IVH (grade ≥3 per the Papile classification).12 The rest were considered normal or having mild degrees of injury. Cerebellar hemorrhage is not formally included in the score; however, we documented its presence.
TEA MRIs were interpreted using the score of Kidokoro et al.5 As our objective was to examine how early detection of WMI was related to a systematic assessment of its sequelae at TEA, we considered the WMI score as well as the global assessment score. WMI is categorized into 4 grades defined as no WMI, mild, moderate, or severe WMI. Five variables are assessed to assign a score for WMI: 1) cystic degeneration, 2) focal signal abnormalities, 3) delayed myelination, 4) dilated lateral ventricles (a ventricular diameter of >7.5 mm is considered relevant, and >10 mm, severe), and 5) reduction of WM volume. Other than WM, scores are assigned for regional injury pertaining to cortical gray matter, deep gray matter, and the cerebellum. A global score of brain injury is assigned by summing all the regional subscores. Four categories of injury are possible: normal, mild, moderate, and severe.
MRIs were interpreted and reported by board-certified neuroradiologists and scored by research clinicians (S.R., G.C.-C.) proficient in neonatal brain MRIs and the use of the 2 scores. Any divergence was discussed with the experts (T.E.I., C.E.) on the project. CUS, performed routinely during NICU stay, and the “worst result” sonography from day 7 to 1-month PMA were noted.
Clinical Data Collection
Demographic and clinical characteristics of all participants were collected from the electronic medical record. Data from CUS, which was performed routinely during the NICU stay, were also gathered to document the highest grades of IVH, WMI, and cerebellar hemorrhage.
Data Analysis
We reported descriptive statistics as means (SD), medians (range), and proportions. We first compared early and TEA MR imaging scores using contingency tables to perform χ2 tests. Because some categories included very small numbers, we dichotomized the severity categories (normal-to-mild versus moderate-to-severe) and performed Fisher exact tests to assess the relationship between early and TEA classifications. We further examined the evolution of the 4 WMI severity categories from early to TEA MRIs graphically. All analyses were performed in SPSS Statistics, Version 28.0 (IBM), and P values <.05 were considered statistically significant.
RESULTS
The cohort consisted of 30 infants, 16 males (53.3%) (Table 1) with a mean gestational age of 28.7 weeks and a median birthweight of 1220 g. All participants were assessed by CUS as per unit protocol, and all participants had satisfactory MR imaging findings at both early and TEA time points. The early MRIs were performed at an average of 33.0 (SD, 1.4) weeks, and the TEA MRIs, at 38.6 (SD, 1.4) weeks’ PMA.
Characteristics of the study participants
Routine clinical CUS identified 5 cases of IVH with dilation (16.7%), 3 infants with cerebellar hemorrhages (10.0%), and 5 with WMI (16.7%). Overall, CUS identified 7 cases with moderate-to-severe injury on global assessment, while early MR imaging identified 11 such infants. MR imaging detected more WMI than CUS (8 versus 5 cases with moderate-severe degrees). Comparison of systematic assessments of early and term MR imaging is shown in Tables 2 and 3. On early MR imaging, 8 infants had moderate-to-severe WMI (26.6%), and 11 met the criteria for moderate-to-severe injury on global assessment (36.7%). By TEA, there were 10 infants with moderate-to-severe WMI (33.3%) and 11 with moderate-to-severe scores for global assessment (36.7%). There was a strong association between the early and TEA WMI severity classifications (P = .007); 81.8% of infants with normal-to-minimal WMI on early MR imaging had a normal-to-mild WMI score at TEA, while 75.0% of infants with early moderate-to-severe WMI remained in the moderate-to-severe WMI risk category at TEA. The relationship between the 2 assessment time points was even stronger when considering the global severity score (P < .001); 94.7% of infants remained in the normal-to-mild overall risk category from early to TEA assessments, and 90.1% remained in the moderate-to-severe overall risk category.
WMI on early-versus-TEA MR imaging
Global assessments on early-versus-TEA MR imaging
When we further examined the sequential assessments of WMI, it appeared that the early WMI scoring predominantly focused on delineating WM lesions themselves, whereas the TEA WMI scoring accounted for the consequences of WMI, even when WM lesions may have themselves become less appreciable (Fig 1). The DWI sequences showed restricted diffusion in 2 cases (of the 30) in the early-acquired MR imaging. Because diffusion restriction is expected in only the acute stages of injury, subsequent imaging at TEA showed normalization of diffusion as an expected evolution. There was no instance of newly detected WMI or increased lesion severity noted on TEA compared with early MR imaging.
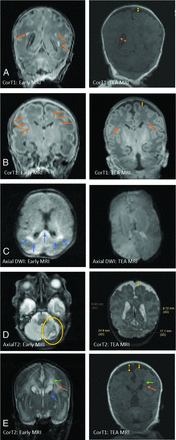
WMI as appreciated in early (1T scanner) versus TEA MR imaging (3T scanner) in different cases. A, The image on the left shows an early MR imaging and the extent of WMI (orange arrows) and its evolution at TEA (image on the right) in which there is an absence of visible WM lesions. WMI sequelae can be appreciated as volume loss, with arrows indicating a dilated ventricle and enlarged subarachnoid space (yellow arrow). B, Early T1 images show severe WMI (orange arrows) with reduced conspicuity at TEA but with some volume loss. C, Early MR imaging at day 3 of life shows diffusion restriction in WM (blue arrows), which has resolved at TEA. D, Isolated unilateral cerebellar hemorrhage (encircled in yellow) with eventual disparity in cerebellar hemispheric sizes and bilateral cerebral WM volume loss at TEA. E, Early IVH (blue arrow) with dilation resulting in periventricular WMI (orange arrows) with cyst formation (green arrows) and volume loss (yellow arrows) at TEA.
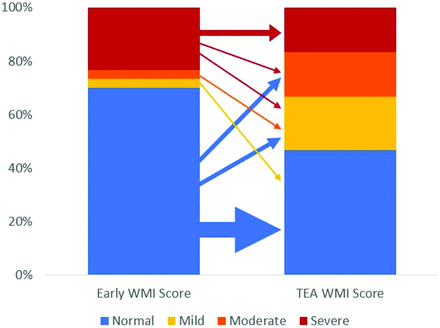
The detailed categorization of WMI severity remained overall stable from early-to-TEA MRIs (Fig 2). Most of the 21 infants with no identified WMI on early MR imaging remained in the normal category at TEA (61.9%), and none were diagnosed with severe WMI. Of these 21 infants, 8 were, however, diagnosed with mild (19.0%) or moderate (19.0%) WMI at TEA. Similarly, most of the 7 infants with early severe WMI remained in the severe category at TEA (71.4%), and none had a normal score. Two infants fell in the remaining severity categories, with some overlap.
Evolution of WMI severity categories from early to TEA MRI. The thickness of arrows is proportional to the number of cases.
DISCUSSION
The application of MR imaging in the preterm infant to evaluate brain injury using systematic scoring systems enables clinicians to analyze images in a systematic manner. A comparison between the 2 commonly used scoring systems for WMI in the preterm infant that are used at differing time points has not been undertaken. In our cohort of 30 preterm infants, early MR imaging detected WMI effectively and correlated highly with the scoring system at TEA that included the sequelae of WMI (Fig 2). The exceptions, those who had TEA scores of higher WMI severity than their corresponding early severity categories, had high grades of IVH, which accounted for the higher TEA score, but no visualized WMI on their early scans. Moreover, the excellent correlation between the global assessment of injury at the 2 time points suggests that MR imaging performed either early or at TEA has prognostic relevance. Individual units may choose to decide the optimal time of imaging according to the availability of resources and the clinical course of the infant. While early imaging may be crucial to inform the next steps, TEA MR imaging exemplifies consequences and evolution of injury in the developing brain.
Several mechanisms resulting in dysmaturity of WM with reduced brain volumes, reflected in TEA MR imaging, as a sequela to IVH have been described in the literature.3,8 The global severity scores, which considered IVH and posthemorrhagic ventricular dilation, were highly correlated. Two infants differed in their severity categories between the early and TEA assessments, one of whom had an isolated, severe cerebellar hemorrhage, which categorized the infant as having moderate injury in the TEA score, but not according to the early score because cerebellar hemorrhage is not included in the scoring system of Miller et al.7
A single infant in our cohort had parenchymal echogenicity detected by sonography, which was not appreciated in the early MR imaging. The gestational age at birth for this infant was 25 weeks 6 days and the echogenicity in the CUS persisted up to the third week of life. The early MR imaging performed at 32 weeks’ PMA (7 weeks of life) detected sequelae of IVH but not WMI. The TEA WMI score suggested moderate injury. Thus, an MR imaging at 30–34 weeks might not be early enough when considering infants of extremely low gestational ages who have sustained WMI early. The ideal definition of “early” in terms of PMA remains to be determined, with consideration for the feasibility of early imaging at low gestational ages when very preterm infants are likely to experience considerable clinical instability, and families, increased stress. Especially considering research involving early imaging, parents need some additional time to be approached for discussions.
It is also useful to consider that the timing around which the pre-oligodendrocytes are most vulnerable to injury is around 28 weeks’ gestation, resulting in the peak of WMI.3 Imaging in the first 2 weeks after birth may thus appear to be a reasonable goal in terms of balancing clinical stability and capturing early injury.13 Repeat imaging may be considered if there are concerns of subsequent WMI due to the NICU course. Early detection of injury would offer an opportunity for higher-intensity and targeted developmental interventions for the high-risk preterm infants and would give essential information regarding evolving neuropathology and research.14
The limitations of this study must be considered, including a small sample size and a low number of cases, particularly in the higher severity categories. The cohort, however, is temporally close and from the same NICU; thus, there is homogeneity in extraneous and possibly confounding factors affecting brain growth and maturation, like nutritional management15 and other aspects of the NICU environment.16 All infants received developmental support available in the NICU from parents, nurses, and allied health professionals, including occupational and physiotherapy.
The scoring systems used at both time points, though providing systematic approaches that have been used in clinical and research settings, are not validated across large populations and with long-term outcomes. The MRIs at the 2 time points have been obtained using different MR imaging systems (1T versus 3T systems). Our team routinely uses the 1T in-NICU MR imaging system for clinical and research scans. The 1T system is equipped for standard sequence acquisition, including T1- and T2-weighted imaging and diffusion-weighted imaging but not for spectroscopy, vascular, and tensor imaging. Although appreciation of subtle structural abnormalities may be different in a system with a weaker magnet, our data show that >90% of MRIs performed in the 1T system do not require follow-up imaging in a 3T unit for this purpose.11 In our study, the lesions visualized in the early scans acquired with the 1T system were less appreciated at TEA due to expected temporal evolution of the lesions.7 Most important, there did not appear to be lesions that were missed on the 1T system.
CONCLUSIONS
Brain MR imaging in infants born preterm can accurately define brain injury, particularly WMI. Although the optimal timing to image the neonate brain remains to be determined, both early (30–34 weeks) and TEA MR imaging appear independently sensitive for WMI and especially for global assessment of injury when analyzed with systematic assessment tools. Early MR imaging should be considered in this vulnerable cohort, and performing imaging in the first 2 weeks of life may be a reasonable goal, depending on the gestational age at birth, clinical stability, and family circumstances. While earlier imaging may capture WM lesions that later resolve yet remain of clinical relevance, TEA MR imaging may offer insight into the consequences of WMI on the developing brain. How information from each time point may be best used and may potentially guide implementation of early intervention measures should be further studied.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received September 7, 2023.
- Accepted after revision November 15, 2023.
- © 2024 by American Journal of Neuroradiology