Abstract
BACKGROUND AND PURPOSE: Given the increased use of stereotactic radiosurgical thalamotomy and other ablative therapies for tremor, new biomarkers are needed to improve outcomes. Using resting-state fMRI and MR tractography, we hypothesized that a “connectome fingerprint” can predict tremor outcomes and potentially serve as a targeting biomarker for stereotactic radiosurgical thalamotomy.
MATERIALS AND METHODS: We evaluated 27 patients who underwent unilateral stereotactic radiosurgical thalamotomy for essential tremor or tremor-predominant Parkinson disease. Percentage postoperative improvement in the contralateral limb Fahn‐Tolosa‐Marin Clinical Tremor Rating Scale (TRS) was the primary end point. Connectome-style resting-state fMRI and MR tractography were performed before stereotactic radiosurgery. Using the final lesion volume as a seed, “connectivity fingerprints” representing ideal connectivity maps were generated as whole-brain R-maps using a voxelwise nonparametric Spearman correlation. A leave-one-out cross-validation was performed using the generated R-maps.
RESULTS: The mean improvement in the contralateral tremor score was 55.1% (SD, 38.9%) at a mean follow-up of 10.0 (SD, 5.0) months. Structural connectivity correlated with contralateral TRS improvement (r = 0.52; P = .006) and explained 27.0% of the variance in outcome. Functional connectivity correlated with contralateral TRS improvement (r = 0.50; P = .008) and explained 25.0% of the variance in outcome. Nodes most correlated with tremor improvement corresponded to areas of known network dysfunction in tremor, including the cerebello-thalamo-cortical pathway and the primary and extrastriate visual cortices.
CONCLUSIONS: Stereotactic radiosurgical targets with a distinct connectivity profile predict improvement in tremor after treatment. Such connectomic fingerprints show promise for developing patient-specific biomarkers to guide therapy with stereotactic radiosurgical thalamotomy.
ABBREVIATIONS:
- BOLD
- blood oxygen level–dependent MRI
- DBS
- deep brain stimulation
- DRTT
- dentato-rubro-thalamic tract
- SMS
- simultaneous multislice
- SRS
- stereotactic radiosurgery
- TRS
- Fahn‐Tolosa‐Marin Clinical Tremor Rating Scale
- VIM
- ventral intermediate nucleus
Tremor is a debilitating neurologic condition that is seen with multiple disorders, most commonly in essential tremor and Parkinson’s disease.1 Patients who are refractory to pharmacologic therapies are potentially candidates for surgical intervention. Deep brain stimulation (DBS) is the current criterion standard surgical treatment; however, not all patients are candidates or wish to undergo DBS. Recently, there has been a resurgence in ablative therapies using incisionless ablative techniques such as stereotactic radiosurgery (SRS) and MR imaging–guided focused ultrasound. While ablative treatment mechanisms might overlap with DBS, little is known on the connectomics of SRS and tremor improvement.
In contrast to traditional “localizationist” models of the brain, a more recent shift to a network, or “connectomic,” model has greatly enhanced our understanding of brain function and pathology.2 Such a shift has also occurred in the realm of neuromodulation with recognition of many disorders as “circuitopathies”3 and the need for targeted network surgery, or “connectomic surgery.”4 The connectomic model, using MR tractography as a measure of structural connectivity and resting-state fMRI as a measure of functional connectivity, has been previously applied to better understand treatment effects in DBS surgery.5⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓-22 In treatment of tremor, this has led to the consolidation of the role of the cerebello-thalamo-cortical sensorimotor network in explaining many previously reported “sweet spots” in the DBS literature.5⇓⇓⇓⇓⇓⇓⇓⇓-14
To date, there has been limited application of the connectomic model to SRS in tremor. Using a combination of resting-state functional MR imaging and MR tractography, we explored whether the therapeutic network for SRS in treatment of tremor displays a “connectome fingerprint” that correlates with tremor improvement. Identification of such a network may provide further insight into the mechanism of tremor improvement and guide targeting in SRS. We also hypothesized that the similarity of any patient's individual connectivity map to the connectome fingerprint of tremor improvement will predict his or her measured tremor improvement, which may establish feasibility for the potential of connectivity fingerprints to guide SRS targeting.
MATERIALS AND METHODS
This prospective clinical trial was approved by the University of Alabama at Birmingham institutional review board. All patients gave verbal and written consent. The trial was registered at https://www.clinicaltrials.gov/ under trial NCT03305588. Subjects were recruited in collaboration with the University of Alabama at Birmingham Neurology, Neurosurgery, Movement Disorders, and Radiation Oncology Departments. Patients with medically refractory essential tremor or tremor-dominant Parkinson disease older than 18 years of age with Eastern Cooperative Oncology Group Performance Status of ≤2 were included. Patients were not candidates for DBS on the basis of either medical/surgical comorbidities or by their own choice. Patients were ineligible if they had prior brain radiosurgery or therapeutic brain radiation therapy or if there was a contraindication to MR imaging.
Imaging Protocol
Before thalamotomy, patients were scanned on a 3T Magnetom Prisma (Siemens) scanner with a high-performance, 80 mT/m gradient system and a 64-channel head/neck coil. Patients’ heads were tightly packed in the head coil to minimize motion.
Diffusion imaging was obtained with a spin-echo EPI sequence using simultaneous multislice (SMS) excitation. A total of 64 diffusion directions were obtained with a b-value = 1500 s/mm2 plus 6 B0 volumes without diffusion-weighting. The diffusion scan was repeated with an opposing phase-encoding direction for distortion correction. Additional relevant parameters include TR = 3.3 seconds, TE = 90 ms, SMS factor = 4, in-plane acceleration = 2, phase partial Fourier = 6/8, receiver bandwidth = 1232 Hz/px, echo spacing = 0.94 ms, EPI factor = 140, in-plane resolution = 1.5 × 1.5 mm, section thickness = 1.5 mm, with scan time = 4 minutes and 21 seconds per acquisition.
A connectome-style resting-state fMRI was acquired with a multiecho blood oxygen level–dependent (BOLD) gradient-echo EPI sequence using SMS excitation. The temporal SNR of BOLD imaging is generally lower in subcortical regions due to many factors, including the distance from the receive coil and increased physiologic noise. To address this issue, we used a multiecho BOLD approach that significantly improves subcortical connectivity measures.23 Additionally, the use of a reduced TR with high spatial resolution and a long, connectome-style BOLD acquisition has all been shown to improve the statistical analysis of resting-state fMRI data. Relevant parameters include the following: TR = 1.5 seconds, echo times = 12.4, 34.3, and 56.2 ms, flip angle = 65°, SMS factor = 4, in-plane acceleration = 2, phase partial Fourier = 6/8, receiver bandwidth = 1850 Hz/px, echo spacing = 0.66 ms, EPI factor = 81, in-plane resolution = 2.0 × 2.0 mm, section thickness = 2.0 mm. The scan was repeated with opposing phase-encoding directions to account for directional bias. A total of 1180 measurements were obtained with a total scan time of 31 minutes and 28 seconds.
A T1-weighted MPRAGE sequence was used for structural registration and performed before SRS. At 3 months after SRS, 3D T1-weighted images were repeated with intravenous contrast. See the Online Supplemental Data for sequence details.
SRS Procedure
Frameless SRS was performed using a thermoplastic mask with an open face molded to the patient’s head. Plans were created using the Eclipse treatment planning system (Varian Medical Systems), and the dose was calculated using either the Analytical Anisotropic Algorithm or AcurosXB algorithm (Varian Eclipse Treatment Planning System) with grid spacing of 1 mm. Patients were treated using an Edge (Varian) linear accelerator equipped with a high-definition multileaf collimator and a 10 MV flattening-filter-free beam by using the virtual cone technique.24 Optical surface guidance was used to monitor the patient position during treatment.25 For full details of the procedure, see the Online Supplemental Data .
Clinical Testing
Before SRS, a baseline Fahn‐Tolosa‐Marin Clinical Tremor Rating Scale (TRS) was assessed. The TRS was repeated at each follow-up assessment after SRS, and the last documented TRS score was used for analysis. TRS was assessed by a single examiner who was blinded to the imaging results at the time of assessment. The primary outcome measure for the study was a percentage decrease in contralateral upper and lower body tremors, measured as the sum of the lateralized TRS scores from Part I on the body side opposite of the treatment hemisphere. Any disease-related medications were maintained at preoperative doses for follow-up.
Image Preprocessing
The postoperative postcontrast T1-weighted images were coregistered to the preoperative T1-weighted images using Advanced Normalization Tools (http://stnava.github.io/ANTs/). The final lesion location was manually segmented as the enhancing region on the 3-month postoperative postcontrast T1-weighted images.
The diffusion data underwent eddy current correction, section-to-volume movement correction, and outlier replacement in FSL “eddy_cuda” (https://git.fmrib.ox.ac.uk/fsl/conda/fsl-eddy-cuda) on a custom-built Linux workstation using 3 NVIDIA Quadro P5000 GPUs. Susceptibility-induced distortions were corrected with FSL “topup” (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/topup) using the repeat acquisitions in opposing phase-encoding directions.26 FSL “BEDPOSTX_GPU” (https://users.fmrib.ox.ac.uk/∼moisesf/Bedpostx_GPU/) was used to estimate the orientation distribution functions with 3 fiber orientations modeled per voxel. The diffusion data were coregistered to the T1 MPRAGE using a boundary-based registration implemented in FreeSurfer (http://surfer.nmr.mgh.harvard.edu). An exclusion mask was generated to include the ventricles and contralateral hemisphere white matter.
The multiecho BOLD data were initially preprocessed in AFNI, Version 22.0.06 (https://afni.nimh.nih.gov/). Preprocessing steps included realignment, spatial smoothing of 4-mm full width at half maximum, and coregistration to the T1 MPRAGE. Denoising of the data was performed with multiecho independent component analysis using TE-dependent analysis implemented in AFNI. Last, motion censoring (framewise displacement of >0.3 mm) and outlier detection were performed using default values in AFNI. Global mean signal regression and regression of motion parameters were also performed.
Connectome Generation
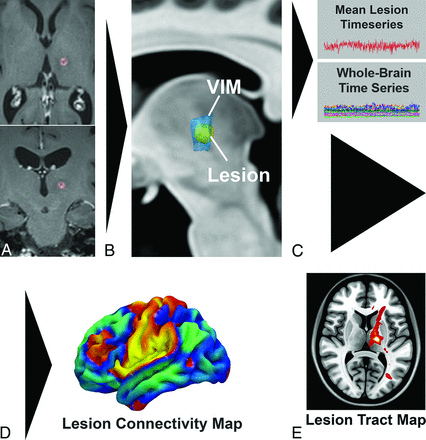
Probabilistic tractography was performed using “probtrackx2_gpu” (https://users.fmrib.ox.ac.uk/∼moisesf/Probtrackx_GPU/index.html) from the FMRIB Software Library, Version 6.0.3 (http://fsl.fmrib.ox.ac.uk) in each of the subjects, with 250,000 samples, a curvature threshold of 0.2, modified Euler streamlining (https://mycareerwise.com/programming/category/numerical-analysis/modified-euler-method), and a step length of 0.5 mm. A region-of-avoidance was added to exclude the internal capsule and deep white matter of the contralateral hemisphere. Because no plausible fibers or existing evidence implicates tracts connecting the thalamus with the contralateral hemisphere via the capsular fibers, such connections are most likely spurious fibers and their inclusion leads to an artificial increase in directions of freedom with resulting inflation of P values. Probabilistic tractography was performed using the segmented lesion as the seed region to generate “lesion tract maps” of all tracts passing through the lesion (Fig 1).
Pipeline for generation of a single-subject lesion connectivity map and lesion tract map (A and B). The lesion is segmented from the posttreatment MR imaging (C). The mean BOLD time-series for the lesion is extracted and correlated to all other brain voxels. D, The resulting t-score map is a patient-specific lesion connectivity map. E, The lesion is used as a seed region to generate a patient-specific lesion tract map representing the probability of all streamlines connected to the lesion.
The preprocessed BOLD resting-state fMRI data underwent a seed-based correlation analysis across the entire brain for each subject using the segmented lesion as the seed point to generate a whole-brain “lesion connectivity map” (Fig 1). The resulting t-maps were used for subsequent analysis.
The structural images, lesion connectivity maps, and lesion tract maps were then normalized into Montreal Neurological Institute template space, as described in the Online Supplemental Data. The right hemisphere lesion connectivity maps and lesion tract maps were nonlinearly flipped to the left hemisphere for comparison across the cohort.
Connectome Analysis
To determine the relationship between structural connectivity and contralateral tremor improvement, we generated an ideal tract fingerprint for the cohort. First, the individual lesion tract maps for each subject were correlated with percentage improvement in the contralateral tremor to generate a Spearman rank-correlation coefficient on a voxel-by-voxel basis. The resultant group R-map, or tract fingerprint, represents an optimal voxel-by-voxel tract map for contralateral tremor improvement.
To assess the significance of the tract fingerprint in predicting outcomes, we performed a leave-one-out cross-validation. An R-map was created by correlating tracts traversing the lesion with the contralateral TRS improvement using all patients except one, withheld for validation. The probabilistic tract map from the left-out patient was then used to calculate spatial similarity (measured via the Fisher z-transformed spatial correlation coefficient) with the tract fingerprint generated from the remainder of the cohort. The similarity index for each left-out subject was then correlated versus the measured tremor improvement using the Spearman correlation.
Next, the process was repeated using the lesion connectivity map generated from the resting-state fMRI for each individual subject to create a functional fingerprint.
Statistics
Demographic data were expressed as mean, range, and SD, as appropriate. The primary outcome measure was the percentage change in the contralateral TRS. Correlation of lesion volume and contralateral TRS improvement was assessed with a nonparametric Spearman correlation. Statistical significance was considered as P < .05.
RESULTS
Demographic and Clinical Results
Forty total patients were enrolled in the clinical trial. Three died of unrelated causes before follow-up, 1 declined treatment, 3 patients were not eligible for 3T MR imaging, and 6 patients were lost to follow-up for their imaging and/or clinical assessment, leaving 27 patients for analysis. Demographic and group outcome data are listed in the Table. Twenty-three (85.2%) patients underwent left thalamotomy, while 4 (14.8%) had right thalamotomy. Twenty patients (74.0%) had a ≥50% reduction in contralateral tremor, while 7 patients (26.0%) had <50% reduction in contralateral tremor. The mean improvement in the contralateral tremor score was 55.1% (SD, 38.9%). All patients had at least 6 months of clinical follow-up with the mean duration of follow-up being 10.0 (SD, 5.0) months. Adverse events determined to be related to radiosurgery included 1 subject with grade I sensory deficits consisting of numbness in the left first and second digits and left-sided mouth and buccal mucosa dysesthesia occurring 10 months after radiosurgery. Two subjects experienced grade II radiation necrosis consistent with hyper-response. One of these subjects experienced right-foot drag, right-hand grip weakness, right-arm proprioceptive loss, mild expressive aphasia, bilateral lip paresthesia, and left-sided headache occurring 7 months after radiosurgery. One subject experienced falls, dysarthria, right-arm and right-leg weakness, and incoordination occurring 11 months after radiosurgery. Both subjects with grade II radionecrosis reported near-resolution of tremor at 4 months after radiosurgery. All 3 subjects with radiosurgery-related adverse events were treated with tapered corticosteroids and bevacizumab infusions. There were no grade III–V adverse events related to radiosurgery.
Demographic and clinical outcome data
Structural Connectome Analysis
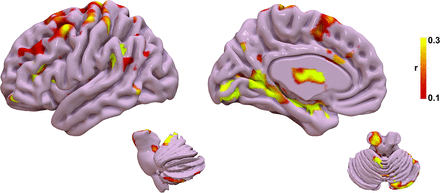
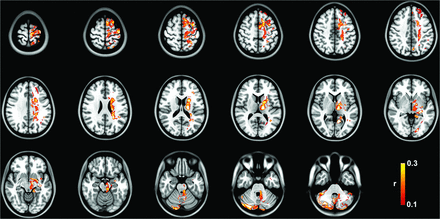
The tract R-map, or tract fingerprint, is shown in Fig 2. The cerebello-thalamo-cortical somatosensory network is a primary component of the affected network, including the primary somatosensory cortex, supplementary motor area, premotor cortex, ventral thalamic nuclei, and cerebellum—primarily cerebellar lobules IV, V, VI, and VIIIB, all areas implicated in the cerebellar somatosensory network.27 Additional connectivity to temporal, occipital, and prefrontal areas is also present. The tract pathways correlated to tremor improvement (Fig 3) show primary involvement of the dentato-rubro-thalamic tract (DRTT); thalamocortical tracts to the primary sensorimotor, supplementary motor area, and prefrontal cortex; as well as the pallidothalamic tracts.
Tract fingerprint representing the ideal tract connectivity pattern for tremor improvement. Commonly implicated areas of abnormality in tremor are identified, including the cerebello-thalamo-cortical motor network, as well as striate and extrastriate cortical regions.
Group tract R-map of pathways most correlated with tremor improvement.
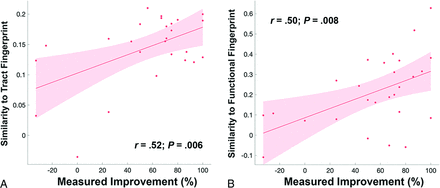
Results of the leave-one-out cross-validation of the tractography are shown in Fig 4A. The structural connectivity correlated with measured improvement in contralateral TRS (r = 0.52; P = .006) and could explain 27.0% of the variance in outcome.
A, Leave-one-out cross-validation of the tract fingerprint shows greater similarity of the individual’s lesion tract map to the fingerprint map predicted measured improvement in tremor (r = 0.52; P = .006). B, Leave-one-out cross-validation of the functional fingerprint also shows that greater similarity of the individual’s lesion functional map to the fingerprint map predicted measured improvement in tremor (r = 0.50; P = .008).
Functional Connectome Analysis
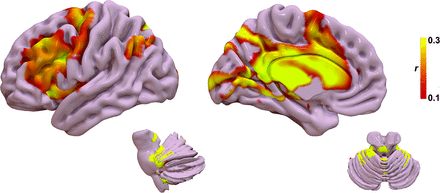
The functional R-map, or functional fingerprint, is shown in Fig 5. The functional connectivity data further corroborate the structural connectivity, showing a correlation with cerebello-thalamo-cortical somatosensory network, in particular the primary somatosensory cortex, ventral thalamic nuclei, and cerebellar lobules IV, V, VI, and VIIIB. Additional connectivity to temporal, occipital, and prefrontal areas is also present. Results for the leave-one-out cross-validation of the functional connectivity are shown in Fig 4B. The functional connectivity correlated with measured improvement in contralateral TRS (r = 0.50; P = .008) and could explain 25.0% of the variance in outcome.
Functional fingerprint representing the ideal tract connectivity pattern for tremor improvement. Similar to the tract fingerprint, commonly implicated areas of abnormality in tremor are identified, including the cerebello-thalamo-cortical motor network as well as the striate and extrastriate cortical regions.
DISCUSSION
In this study, we show that improvement in tremor after SRS thalamotomy is significantly correlated with a distinct pattern of structural and functional connectivity, primarily via the cerebello-thalamo-cortical pathway. This network has been previously demonstrated to be an important driver in tremor pathophysiology and a target for other forms of neuromodulation, such as DBS. Our findings indicate that a significant amount of variance in tremor outcomes after SRS are driven by an identifiable network topology, which may serve as a new therapeutic biomarker for SRS targeting. As opposed to other forms of neuromodulation, such as DBS, there are no intraprocedural biomarkers to validate target selection in SRS. By application of the concept of connectomic surgery,12 we have shown the potential of a lesion connectome fingerprint to predict tremor improvement from a given SRS target using both MR tractography and resting-state fMRI. Our approach may enhance network-specific targeting in SRS and provide patient-specific biomarkers.
The optimal target for neuromodulation of tremor has been debated for decades. Most commonly proposed targets have been the ventral intermediate nucleus (VIM) and the posterior subthalamic area, including the caudal zona incerta, prelemniscal radiations, and subthalamic nucleus.11 More recently, connectivity studies in DBS have suggested that these targets are unified by a common network and all targets overlap with the DRTT.7,14 The role of the DRTT in tremor improvement has been well-demonstrated in DBS,5⇓⇓⇓⇓⇓⇓⇓⇓-14,28⇓⇓⇓⇓⇓-34 but limited data exist on the use of tractography in surgical planning for SRS.35,36 Historically, the DRTT is defined by efferent cerebellar fibers originating in the dentate nucleus of the cerebellum that traverse superiorly through the ipsilateral superior cerebellar peduncle, decussate in the midbrain, pass the red nucleus (but without synapsing in the red nucleus), en route to the contralateral VIM and posterior vetralis oralis nucleus.37,38 Tracts ultimately reach the contralateral primary motor cortex and premotor/supplementary motor cortex from the VIM and vetralis oralis nucleus, respectively. More recently, a small number of DRTT fibers have been shown to follow a similar path but without decussation in the midbrain and extend to the ipsilateral thalamus; however, these tracts likely have less of a role in motor function.37,38 Our study adds further evidence to the role of the DRTT in the surgical treatment of tremor, particularly implicating a similar therapeutic target in SRS as seen in DBS.
From an anatomic perspective, nodes within the fingerprint maps correspond to areas known to exhibit network abnormalities in tremor. First, the fingerprint network distribution agrees with established network abnormalities attributed to the development of tremor via tremor-specific frequency synchronization within multiple nodes in the cerebello-thalamo-cortical pathway, including the cerebellum, thalamus, primary sensorimotor cortex, supplementary motor cortex, and premotor cortex.39,40 Similar to prior work in DBS for essential tremor, we found that a lesion network fingerprint that includes the motor and supplementary motor networks also correlates with tremor improvement in SRS.7,14 Additionally, there was correlation with cerebellar lobules IV, V, and VI, which are regions previously shown to be abnormal in essential tremor and part of the sensorimotor network.41 Taken together, our results suggest that the therapeutic network involved in SRS thalamotomy provides evidence for targeting biomarkers similar to those in the existing DBS literature.
Most interesting, we also found structural and functional connectivity to the primary and extrastriate visual cortex as part of the tremor connectome fingerprint. While not historically considered a major part of tremor pathogenesis, the primary and associative visual cortices have been the subject of more recent investigation of the role of the visuospatial network in tremor augmentation. Archer et al42 showed that visual feedback exacerbates tremor severity in essential tremor and functional changes in extrastriate areas correlate with the worsening of tremor. Likewise, postsurgical alterations in brain connectivity have been observed in the visual cortex that correlated with tremor improvement in MR imaging–guided focused ultrasound43 and SRS;44 however, direct activation of this region was not observed with active DBS.45 Pretreatment functional connectivity between the ventrolateral thalamus and visual association areas has also been shown as a predictor of tremor improvement after SRS.46 Further studies are needed to understand the true causal effect of visual network connectivity versus a coincident change in addition to the cerebello-thalamo-cortical motor network.
Several limitations to our study are noteworthy. Although tremor outcomes were gathered prospectively, the connectomics analyses were applied in retrospect. Thus, future studies would benefit from examining the effects of prospective targeting based on the proposed biomarkers. SRS thalamotomy was effective for tremor and well-tolerated overall. Larger cohorts, however, are needed to expand on these results and better assess the frequency of potential adverse effects. Additionally, intersubject variations in the temporal evolution of the lesion may result in fluctuation of the clinical response and are relatively unpredictable pre-SRS. We chose to use the 3-month post-SRS examination because this was thought to most accurately represent the intended target location with less variability among patients. Last, there are inherent limitations to MR tractography and resting-state fMRI that have been well-described.6,31,47 We used a robust probabilistic tracking approach and a lengthy fMRI acquisition to increase the robustness of our connectome modeling; however, these protocols may be challenging to implement in routine clinical care, and further studies will be needed to explore reproducibility with less robust data sets.
CONCLUSIONS
Using a connectomic approach, we have shown that SRS targets with a distinct connectivity profile predict posttreatment tremor improvement. Among these are networks implicated in tremor pathogenesis, including the cerebello-thalamo-cortical network and visual networks. The use of such connectomic fingerprints may provide a patient-specific biomarker for SRS thalamotomy; however, validation of this approach for use in targeting will require further studies to prospectively validate these results.
Footnotes
This prospective clinical trial was funded by Varian Medical Systems Inc under the study protocol RAD 1601: EDGE Radiosurgery for Intractable Essential Tremor and Tremor-Dominant Parkinson’s Disease (NCT03305588).
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received September 8, 2022.
- Accepted after revision December 30, 2022.
- © 2023 by American Journal of Neuroradiology