Abstract
BACKGROUND: The Woven EndoBridge device was originally approved to treat intracranial wide-neck saccular bifurcation aneurysms. Recent studies have suggested its use for the treatment of sidewall intracranial aneurysms with variable success.
PURPOSE: Our aim was to evaluate the safety and efficacy of the Woven EndoBridge device for sidewall aneurysms using a meta-analysis of the literature.
DATA SOURCES: We performed a systematic review of all studies including patients treated with the Woven EndoBridge device for sidewall aneurysms from inception until May 2022 on Scopus, EMBASE, MEDLINE, the Web of Science, and the Cochrane Central Register of Controlled Trials.
STUDY SELECTION: Ten studies were selected, and 285 patients with 288 sidewall aneurysms were included.
DATA ANALYSIS: A random-effects meta-analysis of proportions using a generalized linear mixed model was performed as appropriate. Statistical heterogeneity across studies was assessed with I2 statistics.
DATA SYNTHESIS: The adequate occlusion rate at last follow-up was 89% (95% CI, 81%–94%; I2, = 0%), the composite safety outcome was 8% (95% CI, 3%–17%; I2 = 34%), and the mortality rate was 2% (95% CI, 1%–7%; I2 = 0%). Aneurysm width (OR = 0.5; P = .03) was the only significant predictor of complete occlusion.
LIMITATIONS: Given the level of evidence, our results should be interpreted cautiously until confirmation from larger prospective studies is obtained.
CONCLUSIONS: The initial evidence evaluating the use of the Woven EndoBridge device for the treatment of wide-neck sidewall intracranial aneurysms has demonstrated high rates of adequate occlusion with low procedural complications. Our findings favor the consideration of the Woven EndoBridge device as an option for the treatment of sidewall aneurysms.
ABBREVIATIONS:
- GCP
- good clinical practice
- NOS
- Newcastle-Ottawa Quality Assessment Scale
- WEB
- Woven EndoBridge
The endovascular treatment of wide-neck bifurcation aneurysms has prompted the development of new techniques and devices.1⇓-3 As a result, intrasaccular flow disruption with the Woven EndoBridge (WEB; MicroVention) device has emerged as a safe and effective alternative without the requirement of long-term antiplatelet therapy. Good clinical practice (GCP) studies developed in Europe and the United States led to the approval of the WEB device by the US Food and Drug Administration for the treatment of adults with intracranial wide-neck bifurcation aneurysms.4⇓⇓⇓-8 Subsequently, several postmarketing prospective studies with long-term follow-up have confirmed the good long-term efficacy, stability, and safety of the WEB for the treatment of bifurcation aneurysms.9
Following the initial release and experience with the WEB device, several adjustments and innovations to its design and delivery system have been made.10,11 Of note, the device is currently available in smaller sizes with improved visibility. In addition, the delivery system has decreased its profile to 0.017-inch microcatheters for WEB sizes 3–7 mm.12 With these adjustments, the use of the WEB has gradually evolved to include smaller and distally located aneurysms. Furthermore, several published reports have suggested that expanding initial indications might be feasible while maintaining a safe profile.10,13⇓⇓-16
Hence, we sought to evaluate the safety and efficacy of the WEB device for sidewall aneurysms stratified by size and location using our institutional experience and an aggregate meta-analysis of proportions. In addition, we evaluated the predictors of complete occlusion at follow-up using patient-level data.
MATERIALS AND METHODS
Protocol and Guidance
This systematic review used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement to report the search results.17
Search Strategy and Eligibility Criteria
We performed a comprehensive literature search in Scopus, EMBASE, MEDLINE, the Web of Science, and the Cochrane Central Register of Controlled Trials from inception until May 2022. The complete search strategy is provided in the Online Supplemental Data. Articles were included in the analysis if they met the following criteria: 1) randomized clinical trials, nonrandomized trials, and cohort observational studies and case series (≥5 cases) of adult (18 years of age or older) patients with sidewall intracranial aneurysms (outside the traditional indications from the GCP studies4⇓⇓-7) and treated with any of the WEB devices, 2) publication language in English or Spanish, and 3) at least 1 of our prioritized outcomes reported. Case reports, abstracts, commentaries, and reviews were excluded.
Study Selection
The search strategy was applied individually to each database. Two reviewers independently screened all studies by titles and abstracts to identify potentially relevant articles. Finally, the same reviewers accessed the full-text versions and determined their eligibility. Any disagreements were resolved through an initial discussion between the 2 reviewers. A third reviewer was considered an arbitrator if no consensus was reached.
Data Collection Process and Outcomes
Two reviewers independently extracted data from the included studies using a standardized electronic form. We extracted baseline, angioarchitectural, and procedural characteristics. An Excel (Microsoft) datasheet was uniformly sent by e-mail to the first and corresponding authors of the selected studies with aggregated data to extract patient-level data. Finally, the received data sets were merged into a summary database for the patient-level analysis.
The primary efficacy outcome was the proportion of adequate angiographic occlusions at the last follow-up, defined as a Raymond-Roy scale of I–II or a Bicêtre Occlusion Scale of 0, 0′, or 1. Secondary efficacy outcomes included complete occlusion at the last follow-up (defined as a Raymond-Roy of I or a Bicêtre Occlusion Scale of 0 or 0′), immediate complete occlusion, mRS at the last follow-up (a favorable outcome was defined as mRS 0–2), aneurysm retreatment, and the technical success rate. The primary safety outcome was a composite including intraprocedural and postprocedural complications. Intraprocedural complications included thromboembolic events, hemorrhagic events, device-deployment issues, and air embolisms. Postprocedural complications included ischemic and hemorrhagic events. Our secondary safety outcomes were the rate of intraprocedural complications, postprocedural complications, and all-cause mortality.
Risk of Bias and Certainty of the Evidence
Two reviewers used the Newcastle-Ottawa Quality Assessment Scale (NOS) for cohort studies to assess the methodologic quality of the included studies. According to the Cochrane recommendations, we assessed the certainty of the body of evidence from eligible studies in the quantitative synthesis.18 We used the Grading of Recommendation, Assessment, Development, and Evaluation approach.
Data Synthesis
A random-effects meta-analysis of proportions was performed using a generalized linear mixed model to estimate pooled rates and 95% CIs for each prioritized outcome. Statistical heterogeneity across studies was assessed with the I2 test (>50% suggests substantial heterogeneity), while heterogeneity between subgroups was assessed with the Cochran Q test for heterogeneity. We planned further prespecified subgroup analyses by using the available patient-level data for the anatomic territory of the aneurysm (anterior circulation subgroup versus posterior circulation subgroup), the maximal diameter of the aneurysm (<7 versus ≥7 mm), and the rupture status (ruptured versus unruptured). A mixed-effects logistic regression to study the predictors of complete and adequate occlusion with variables selected via backward stepwise regression was performed using the patient-level data.
Institutional Experience
We performed a retrospective review of all patients with sidewall aneurysms who underwent endovascular treatment with the WEB device at our institution between September 2020 and November 2021. Institutional review board (University of Iowa Hospitals & Clinics) approval was obtained. Data on the demographic, clinical, and radiologic characteristics of the patients were collected. The morphologic features of the aneurysm and treatment outcomes were determined.
The indication for endovascular treatment was determined by a multidisciplinary team of neurovascular surgeons and neurointerventionalists. The selection of the WEB in these patients was determined according to the characteristics of the patient and aneurysm when other management options such as primary coiling, stent-assisted coiling, balloon-assisted coiling, flow diversion, and remodeling were deemed not the best treatment option. The procedure and WEB-size selection were performed in the same fashion as previously described and suggested by the manufacturer.14,19 Only when there was a concern for WEB protrusion into the parent vessel aspirin was prescribed for 6 weeks following the procedure. In general, the clinical and imaging follow-ups were performed at 3, 6, and 12 months.
RESULTS
Study Selection and Characteristics
A total of 1025 documents were identified, and 626 duplicates were removed (Online Supplemental Data). As a result of the initial screening by title and abstract, there were 50 potentially eligible documents. Next, in the full-text evaluation, 40 documents were excluded due to the type of included population, the absence of data of interest, or the study design (Online Supplemental Data). Finally, 10 studies were included from the final systematic search. Patient-level data from 104 patients, including 9 patients from our institution (Online Supplemental Data) were available and included.
Seven studies were conducted in Europe,10,12,14,15,20⇓-22 and 3, in the United States.13,16,23 A total of 285 patients (79% female; mean age, 58 years) with 288 sidewall aneurysms (35% ruptured) were included in our aggregate meta-analysis. Most aneurysms were wide-neck (92%). Most were located in the anterior circulation (80%). Of them, the posterior communicating artery (20%), the communicating segment of the internal carotid artery (14%), and the paraophthalmic segment (12%) were the most common locations. From those aneurysms in the posterior circulation (20%), the most common locations were the superior cerebral artery (17%) and the posterior inferior cerebellar artery (17%). Details about the antiplatelet regimens used in each study are summarized in the Online Supplemental Data. The mean follow-up was 10.4 months and ranged from 3.3 to 29.5 months. The characteristics of all studies are presented in the Online Supplemental Data.
Risk of Bias within Studies and Certainty of the Evidence
Using the NOS, we rated 8 studies as high quality and 2 as moderate quality. All the studies earned one point for representativeness of the exposed cohort. Details are shown in the Online Supplemental Data. The certainty of the evidence was assessed for each outcome individually in the overall population. The assessment for each outcome is presented in the summary of findings table (Online Supplemental Data). Publication bias was detected for immediate complete occlusion, favorable clinical outcome, postprocedural complications, and mortality on the basis of funnel plot visualization (Online Supplemental Data).
Synthesis of Results
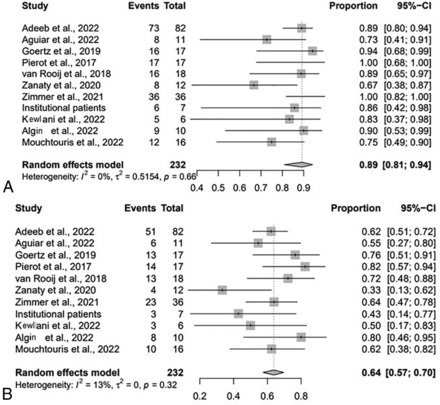
At last-follow up, the rate of adequate occlusion was 89% (95% CI, 81%–94%; I2 = 0%, P = .66) (Fig 1A). The technical success rate for implanting the WEB was 99% (95% CI, 79%–100%; I2 = 0%, P = 1.00). The immediate complete occlusion rate was 37% (95% CI, 30%–43%; I2 = 0%, P = .5), and the complete occlusion rate at last follow-up was 64% (95% CI, 57%–70%; I2 = 13%, P = .32) (Fig 1B). The favorable clinical outcome rate (mRS 0 – 2) was 89% (95% CI, 75%–96%; I2 = 48%, P = .07), and the retreatment rate was 9% (95% CI, 5%–13%; I2 = 0%, P = .82).
Forest plot for adequate (A) and complete (B) occlusion at last follow-up by study.
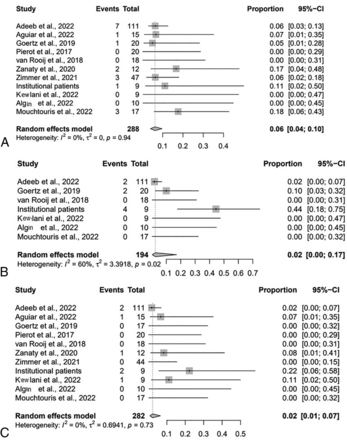
The rate of the composite safety outcome was 8% (95% CI, 3%–17%; I2 = 34%, P = .13) (Fig 2A). The intraprocedural complication rate was 6% (95% CI, 4%–10%; I2 = 0%, P = .94) (Fig 2B); 2% (95% CI, 1%–5%; I2 = 0%, P = 1.00) were thromboembolic complications, 1% (95% CI, 0%–14%; I2 = 0%, P = .91) were hemorrhagic, 1% (95% CI, 0%–3%; I2 = 0%, P = .99) were device-deployment issues, and 1% (95% CI, 0%–4%; I2 = 0%, P = 1.00) were vascular dissections. The postprocedural complication rate was 1% (95% CI, 0%–1%; I2 = 33%, P = .14) (Fig 2C). The all-cause mortality rate was 2% (95% CI, 1%–7%; I2 = 0%, P = .73).
Forest plot for composite safety outcome (A) intraprocedural complications (B), and postprocedural complications (B) by study.
Subgroup Analysis
Subgroup analysis for rates of adequate occlusion was consistent with the rates of the entire cohort for ruptured (68%) and unruptured (80%) sidewall aneurysms (Online Supplemental Data). Similarly, composite safety outcome rates were similar for ruptured (6%) and unruptured (6%) sidewall aneurysms (Online Supplemental Data).
Subgroup analysis for adequate occlusion and the composite safety outcome was consistent for the anterior and posterior circulation (Online Supplemental Data). On subgroup analysis by aneurysm maximal diameter, adequate occlusion and the composite safety outcome were consistent for aneurysms of <7 and ≥7 mm (Online Supplemental Data).
Patient-Level Predictive Analysis
The variables selected using stepwise regression included maximal aneurysm diameter, neck size, height, width, height difference (the difference between the height of the aneurysm and the WEB device [before opening]), and width difference (the difference between the width of the aneurysm and the WEB device [before opening]) (Online Supplemental Data). The aneurysm width (OR = 0.5; 95% CI, 0.26–0.95; P = .03) was the only independent predictor of complete occlusion at the last follow-up. None of the variables included predicted adequate occlusion.
DISCUSSION
While multiple prospective and retrospective studies have demonstrated the safety and effectiveness of the WEB device for intracranial wide-neck bifurcation aneurysms,24⇓⇓-27 only a few studies have reported treatment results with the WEB for sidewall aneurysms. In this meta-analysis of patients treated with the WEB for wide-neck sidewall aneurysms, we found the following: 1) The WEB device has an efficient profile with a high rate of adequate occlusion (89%) at follow-up, and 2) it has a safety profile with a low rate of our composite safety outcome (8%). Furthermore, we determined that the aneurysm width was the main predictor of complete occlusion at the last follow-up in wide-neck sidewall aneurysms.
In the cumulative population of the WEB Clinical Assessment of Intrasaccular Aneurysm Therapy (WEBCAST), French Observatory, and WEBCAST-2 studies (168 patients), complete and adequate occlusion was observed in 52.9% and 79% at 1-year follow-up, respectively.28 Moreover, in the WEB Intrasaccular Therapy (WEB-IT) study, complete and adequate occlusion rates at 1-year follow-up were 53.8% and 84.6%, respectively.4 The rates of complete (64%) and adequate (89%) occlusion at the last follow-up observed in our meta-analysis are comparable with the results from the GCP studies. Furthermore, several meta-analyses have reported similar findings in wide-neck bifurcation aneurysms.29⇓-31 On the other hand, recent meta-analyses of flow diversion for the treatment of sidewall aneurysms have shown pooled complete (range, 69.5%–74.9%)32,33 and adequate occlusion (range, 84.7%–88.9%) rates,33,34 comparable with our findings.
Considering the composite safety outcome of the WEB device for wide-neck sidewall aneurysms, we found an 8% rate of intraprocedural and postprocedural complications. Focusing on the intraprocedural complications, our findings are lower than the 8.4% pooled rate of intraprocedural complications reported by Monteiro et al24 in a recently published meta-analysis evaluating ruptured intracranial aneurysms treated with the WEB device. Furthermore, when we compared our findings with those in a recent meta-analysis that included ruptured and unruptured aneurysms, our hemorrhagic rate was similar to the reported 0.83% rate and our thromboembolic event rate was lower than the 5.6% reported rate.31 Of note, meta-analyses of flow diversion for sidewall aneurysms have shown higher pooled complication rates ranging from 7.8% to 27.1%.32⇓-34 The definition of complications in the studies we included for meta-analysis was inconsistent, so a direct comparison across studies might be limited. Next, considering the postprocedural complications, our findings were significantly lower than the 14% reported by Tau et al35 on a meta-analysis evaluating the WEB for all types of aneurysms and similar to the 1% rate reported in the previously mentioned meta-analysis of ruptured aneurysms.24
Our patient-level data-predictive analysis found that a smaller aneurysm width increased the probability of complete occlusion at the last follow-up after WEB treatment. Nevertheless, although the rest of the variables included in the model were not statistically significant predictors (Online Supplemental Data), the tendency of the association is according to previous reports.36⇓-38 Considering that patient-level data were available for less than half of the patients, a clear limitation was our small number of patients, which was insufficiently powered to detect a modest effect of some of the parameters studied.
Our appraisal of the certainty of the evidence allowed us to assess the quality of the evidence for each of our prioritized outcomes. From this assessment, we have been able to identify limitations of this meta-analysis. First was the methodologic design of the included studies. The retrospective design of all the studies inherently comes with selection bias, and because most did not include direct comparisons with wide-neck bifurcation aneurysms or other treatment strategies, a comparative meta-analysis was not possible. Second, most included studies did not have long-term follow-up periods (>18 months). Third, almost all the evidence from the WEB device is focused on the indications from the GCP studies; therefore, the available literature included in this meta-analysis is limited by studies with small sample sizes, increasing the heterogeneity and lack a standardized assessment of the angiographic parameters. Finally, we did not perform a meta-analysis with adjusted effect sizes for potential covariates due to the limited sample size.
Implications for Clinical Practice
While a detailed characterization of the aneurysm location, angle, size, and morphologic features is fundamental for the best treatment selection, the addition of intrasaccular flow disruption for the treatment of wide-neck sidewall aneurysms expands the neurointerventionalist’s toolbox for the treatment of intracranial aneurysms. Furthermore, with the continuous evolution of the WEB device and its delivery system, its use has become less technically challenging, allowing the continual expansion of its use. The flow-disruption technique can potentially become a valuable treatment selection for hard-to-treat aneurysms for which the standard coil-based methods have limited performance.
CONCLUSIONS
The initial evidence evaluating the use of the WEB for the treatment of wide-neck sidewall intracranial aneurysms has demonstrated high rates of adequate occlusion with low procedural complications. Our findings favor the consideration of the WEB device as an option for the treatment of sidewall aneurysms.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received November 9, 2022.
- Accepted after revision December 18, 2022.
- © 2023 by American Journal of Neuroradiology