Abstract
BACKGROUND AND PURPOSE: CTA can rapidly and accurately detect and localize occlusive disease in patients with ischemic stroke. We have used CTA to assess arterial stenosis and occlusion in an ischemic stroke population arriving at a tertiary stroke center within 24 hours of symptom onset in order to obtain a comprehensive picture of occlusive disease pattern, and to determine the proportion of eligible candidates for endovascular treatment.
MATERIALS AND METHODS: Data from consecutive patients with acute ischemic stroke admitted to a single center between 2003 and 2012, collected in the Acute Stroke Registry and Analysis of Lausanne data base, were retrospectively analyzed. Patients with a diagnostic CTA within 24 hours of symptom onset were selected. Relevant extra- and intracranial pathology, defined as stenosis of ≥50% and occlusions, were registered and classified into 21 prespecified segments.
RESULTS: Of the 2209 included patients (42.1% women; median age, 72 years), 1075 (48.7%) had pathology in and 308 (13.9%) had pathology outside the ischemic territory. In the 50,807 arterial segments available for revision, 1851 (3.6%) abnormal segments were in the ischemic (symptomatic) territory and another 408 (0.8%) were outside it (asymptomatic). In the 1211 patients with ischemic stroke imaged within 6 hours of symptom onset, 40.7% had symptomatic large, proximal occlusions potentially amenable to endovascular therapy.
CONCLUSIONS: CTA in patients with acute ischemic stroke shows large individual variations of occlusion sites and degrees. Approximately half of such patients have no visible occlusive disease, and 40% imaged within 6 hours show large, proximal segment occlusions amenable to endovascular therapy. These findings show the importance of early noninvasive imaging of extra- and intracranial arteries for identifying occlusive disease, planning recanalization strategies, and designing interventional trials.
ABBREVIATIONS:
- AIS
- acute ischemic stroke
- ASTRAL
- Acute Stroke Registry and Analysis of Lausanne
More than 80% of strokes are ischemic, usually caused by large-artery atherosclerosis, cardiac embolism, or cerebral microangiopathy.1 CTA or MR arterial neuroimaging is frequently used at admission2,3 to determine occlusion sites and clot extent and to plan acute recanalization strategies.4⇓⇓–7 CTA is widely available and allows rapid assessment of the entire arterial vasculature from the aortic arch to the vertex. In addition, it accurately depicts arterial occlusive disease with good interrater agreement.8 Most clinically relevant arterial occlusive disease is found in the extra- and intracranial arteries, supporting aortic arch-to-vertex CTA for patients with acute ischemic stroke (AIS).9 Potential drawbacks of CTA are iodinated contrast allergy and radiation exposure.
The purpose of this study was to obtain a comprehensive picture of cerebrovascular occlusive disease in a representative AIS population, using admission CTA, to determine the proportion of eligible candidates for endovascular treatment. Such patients were identified by looking for symptomatic proximal arterial occlusions readily accessible with endovascular devices. Moreover, we aimed at determining the proportion of patients with abnormal segments in ischemic and nonischemic territories and describing the distribution of arterial occlusive disease in extra- versus intracranial vessels, anterior-versus-posterior circulation, and serial (tandem) pathologies.
Materials and Methods
Patients
We used the patients admitted with AIS between January 2003 and December 2012 from the prospectively constructed Acute Stroke Registry and Analysis of Lausanne (ASTRAL), which collects information on all patients with AIS referred to the stroke center and/or intensive care unit of Lausanne University hospital (Centre Hospitalier Universitaire Vaudois) within 24 hours of the last-known-well time.1 We included all consecutive patients who had a diagnostic CTA available for analysis. CTA studies were rated as diagnostic or nondiagnostic by at least 2 authors, and the latter studies were excluded when at least 1 author considered them nondiagnostic (On-line Fig 1). A diagnostic CTA provides a sharp delineation of head and neck vessels, allowing evaluation of partial or complete filling defects. Patients arriving later than 24 hours from symptom onset and presenting with transient ischemic attack, cerebral or subarachnoid hemorrhage, persistent retinal ischemia, amaurosis fugax, and spinal cord ischemia were excluded. Reasons for exclusion were collected.
CTA-based imaging information, demographic data, cardiovascular risk factors, and ischemic side and territory were extracted and analyzed retrospectively. Stroke was categorized according to the TOAST (Trial of ORG 10172 in Acute Stroke Treatment) classification,10 and we added dissection, likely patent foramen ovale–related stroke, and combinations of mechanisms.
Neuroimaging Protocol
All included patients underwent standard AIS imaging, including noncontrast head CT, cerebral perfusion CT, and cervicocerebral CT angiography. In patients undergoing thrombolysis, CTA was acquired immediately before or immediately after the rtPA bolus; therefore, this process made it unlikely that thrombolysis influenced the arterial occlusive pathology. Imaging was performed on a 16–detector row CT scanner (LightSpeed 16 Advantage; GE Healthcare, Milwaukee, Wisconsin) until November 2005 and afterwards on a 64–detector row CT scanner (LightSpeed VCT 64; GE Healthcare). Technical parameters for cerebral and extracranial CTA were the following: multidetector-array in a helical mode, 120 kV(peak), 150–300 mAs, section thickness = 1.25 mm before 2006 and 0.63 mm thereafter, pitch = 0.9:1. Image-acquisition delay depended on the arrival time of a 20-mL contrast bolus test (range, 15–20 seconds). Acquisition was then performed after intravenous administration of 50 mL of iohexol (300 mg/mL of iodine, Accupaque; GE Healthcare) at a rate of 5 mL per second with a power injector (Stellant D CT Injection System; Medrad, Indianola, Pennsylvania) in the arterial phase. Coverage extended from the origin of the aortic arch to the top of the corpus callosum. Delayed phase images were obtained on the brain only.
Image Interpretation
Only CTA images were used for this study. Raw CTA source images in the axial plane were analyzed for focal arterial occlusive disease. Maximum intensity projections were then reconstructed in axial, sagittal, and coronal planes. Curvilinear reconstructions were obtained routinely.
A senior board-certified vascular neurologist (P. Michel) or neuroradiologists (R.A.M. or P. Maeder) with >10 years' experience analyzed the acute CTAs within 7 days after admission. In cases of discordance, images were reviewed by the vascular neurologist and at least 1 neuroradiologist to reach agreement. Interrater agreement was assessed on 100 consecutive patients in the data base by using κ statistics and comparing the CTA interpretations by 1 vascular neurologist (P. Michel) and 1 neuroradiologist (P.J.M.). Both independently assessed the presence or absence of extracranial and intracranial segmental occlusive disease in the proximal (carotid siphon, M1, basilar artery) and distal circulation in the ischemic territory.
Twenty-one arterial segments (counting left and right sides, listed below) were analyzed separately for each patient and graded as normal, stenotic, or occluded. Segments were the following: extracranial vertebral artery (V1–3 segments); intracranial vertebral artery (V4 segment); basilar artery; posterior cerebral artery, P1, P2, and P3 segments; extracranial internal carotid artery; intracranial carotid artery, noting whether T-occlusion was present or not; middle cerebral artery, M1, M2, M3 segments; and anterior cerebral artery, A1, A2, and A3 segments. Imaging data are continuously recorded in the ASTRAL data base.
The term “abnormal” was used for stenotic or occluded segments.
“Occlusion” was defined as the absence of contrast medium filling the examined arterial segment on initial acquisition.11 For extracranial arteries, stenosis was defined by using the NASCET criteria: caliber reduction of ≥50% for vertebral arteries and ≥70% for carotid arteries.12 For intracranial arteries, stenosis was defined by using the Warfarin-Aspirin Symptomatic Intracranial Disease method (ie, ≥50% caliber reduction).13 “Tandem patterns” are defined as arterial occlusive disease affecting both the extra- and intracranial circulation in the same vascular axis. Abnormal segments on CTA were further categorized as symptomatic if the stenosis or occlusion was ipsilateral and proximal to the acute ischemic territory, or asymptomatic, if they were in the nonacute ischemic territory.
To evaluate the number of possible acute mechanical revascularization procedures in readily accessible large arteries, we subjected all occlusions in the extracranial and intracranial carotid arteries, V1–3, V4, basilar artery, P1, A1, M1, and M2 segments to a specific group analysis.
Data Processing and Statistical Analysis
Univariate analysis was performed to compare the characteristics of included and excluded patients by using the Wilcoxon 2-sample test for continuous variables and the χ2 test for categoric variables. P values < .05 were considered significant. All data were processed by using STATA statistical software (Version 13.1, October 30 2013; StataCorp, College Station, Texas). Odds ratios were obtained by using an on-line calculator.14
ASTRAL was approved for scientific use by the institutional ethical commission, which did not require that individual informed consent be obtained.
Results
Of 5022 patients with AIS symptoms who arrived at our institution during the observation period, 2209 patients were enrolled. Reasons for exclusion from ASTRAL were vascular diagnoses other than AIS (n = 2370). These and radiologic reasons for exclusion from the study (n = 443) are detailed in On-line Fig 1.
Baseline characteristics of the included (n = 2209) and excluded (n = 443) patients with AIS are described in Table 1. In univariate comparison, patients with AIS excluded for radiologic reasons were older and more often women and tended to have more hypertension and atrial fibrillation and lower admission NIHSS scores. Stroke onset-to-CT and door-to-CT delays were longer, as expected in this group, because a main reason for study exclusion was a CTA performed after 24 hours of onset.
Patient characteristics and univariate comparison with the patients excluded for radiologic reasonsa
Cervicocerebral CTA analysis of the 2209 enrolled patients yielded 50,807 analyzable arterial segments.
Interrater agreement in the ischemic territory was almost perfect for intracranial proximal occlusive disease (κ = 0.87) and substantial for intracranial distal and extracranial occlusive disease (κ = 0.61 and 0.64, respectively), similar to previously published data.8
One thousand two hundred twenty-six patients (55.5%) had any arterial occlusion or stenosis (ischemic and nonischemic territories combined), while 983 patients (44.5%) did not show relevant occlusive disease (for details see Tables 2 and 3). One thousand seventy-five patients (48.7% of all patients) had such major abnormalities in the ischemic (ie, symptomatic) territory. Three hundred eight patients (13.9% of all patients) had stenosis or occlusion in territories not related to ischemia (ie, asymptomatic abnormalities) (see On-line Tables 1 and 2 for full details).
Distribution of arterial occlusive disease in numbers and rates, given per patient (N = 2209)a
Distribution of occlusive disease for each arterial segmenta
If one considered arterial segments, arterial occlusion or stenosis occurred in 2259 segments (4.5% of all segments) (for details, see Tables 2 and 3); 1851 segmental abnormalities (81.9% of all abnormal segments or 3.6% of all examined segments) were in the ischemic territory (ie, symptomatic). Four hundred eight segmental abnormalities (18.1% of all abnormalities or 0.8% of all examined segments) were in the nonischemic territory (ie, asymptomatic) (see On-line Tables 1 and 2 for full details).
Occlusive disease was most commonly seen in the anterior circulation (76.1% of all abnormal segments). This was more often intracranial than extracranial (30.9% versus 24.6% of all patients).
The proximal MCA (24.7% of all symptomatic abnormalities) was most frequently affected by symptomatic occlusive disease, followed by the extracranial ICA (20.2%) and then the distal MCA (14.5%).
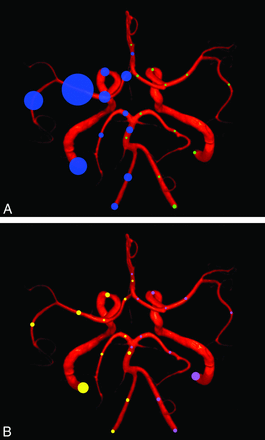
As seen in the tables, occlusion was more frequent than stenosis. Symptomatic tandem patterns (On-line Table 3) were predominantly observed in the anterior circulation (82.1%) in 302 patients (28.1% of all patients with symptomatic abnormalities, 13.7% of all patients in the study). A graphic representation of observed occlusive disease per segment (symptomatic and asymptomatic stenosis or occlusions) is available in Fig 1. A column plot representation of arterial occlusive disease according to localization and type (stenosis versus occlusion) as defined in the “Materials and Methods” section is available in On-line Fig 2. Of patients having a symptomatic intracranial abnormality, 40% had a coexisting extracranial abnormality (tandem pattern). Only 34 of the tandem lesions (10.1% of all tandem lesions) were asymptomatic.
Graphic representation of observed occlusive disease per segment. The area of the circle is proportional to the observed rate. A, Rate of symptomatic (right patient side, blue circles) and asymptomatic arterial occlusions (left patient side, green circles). B, Rate of symptomatic (right patient side, yellow circles) and asymptomatic arterial stenoses (left patient side, pink circles).
In 743 (33%) of the patients with AIS imaged within 24 hours of symptom onset, we found at least 1 symptomatic occlusion in a large, proximal artery and therefore readily accessible to endovascular treatment. These numbers increased to 640 (38.1%) of the 1679 patients imaged within 12 hours, 531 (40.7%) of 1304 imaged within 6 hours, and 480 (42.0%) of 1143 patients imaged within 4.5 hours. Of the patients arriving within 6 hours, the following segments were found occluded on CTA: extracranial ICA (n = 150, 11.5% of patients); V1–V3 (n = 31, 2.4% of patients); intracranial ICA (n = 34, 2.6% of patients); M1 (n = 193, 14.8% of patients); M2 (n = 114, 8.7% of patients); A1 (n = 32, 2.5% of patients); P1 (n = 23, 1.8% of patients); V4 (n = 11, 0.8% of patients); basilar artery (n = 22, 1.7% of patients). This is a total of 610 segments, found in 531 patients.
Discussion
In the largest series to date of patients with AIS undergoing CTA within 24 hours from symptom onset, we found relevant arterial abnormalities in 55.5% of patients, most of which were observed in the ischemic territory. Regarding individual cervical and cerebral arterial segments, only 4.5% were abnormal, again affecting predominantly the ischemic territory. Arterial segments with the highest blood supply (anterior > posterior circulation, larger > smaller arteries) had the most occlusive disease.
Approximately half of the patients with AIS did not have any visible arterial abnormalities on CTA, increasing the chances of a better outcome15 and making endovascular treatment unnecessary. The fact that an overwhelming proportion of arterial segments are patent in AIS (95.5%) is a reminder that there is a great potential for collateral blood supply into the ischemic territory, potentially allowing access of thrombolytic or neuroprotective drugs to the acutely ischemic brain.
Symptomatic arterial abnormalities were more often found in the anterior circulation (76.1% of all abnormal segments, occurring in 81.6% of patients with symptomatic abnormalities), which is consistent with the proportion of blood flow being directed to this part of the cerebral circulation. In addition, there is significantly more arterial occlusive disease in patients with anterior-versus-posterior circulation stroke (see odds ratios in Table 4). This may be related to posterior circulation strokes being more often of microangiopathic origin,16 with less detectable arterial pathology on noninvasive imaging.
Localization of arterial occlusive disease in the ischemic territorya
Patients more often had symptomatic arterial abnormalities in the intra- rather than the extracranial circulation (28.6% versus 20.0% of all patients). This may be because most emboli from the heart and proximal extracranial arteries are too small to occlude the cervical arteries and get blocked only in smaller, intracranial vessels. The finding that the proportion of abnormal arterial segments is lower intracranially (3.8% versus 7.7%, Table 3) is because the intracranial circulation is divided into subsegments. Our results, therefore, show that it is crucial to examine both the extracranial arteries and the circle of Willis in patients with AIS.
Not surprising, symptomatic abnormalities are usually occlusive, whereas asymptomatic abnormalities are typically stenotic, likely explained by a reduced-but-sufficient blood flow through the stenosis. We found a low overall rate (0.7%) of symptomatic intracranial stenosis in our cohort, likely consisting of a combination of atherosclerotic plaques and partially resorbed emboli. A higher rate of intracranial stenosis has been described in Asian patients with ischemic stroke.17
The 40% concurrent extracranial (tandem) pathology observed in patients with a symptomatic intracranial abnormality has practical implications because it may complicate endovascular access. This situation seems to be particularly frequent in the anterior circulation (odds ratio of 5.0 for anterior-versus-posterior tandem lesions in our population).
Rapid endovascular treatment by using predominantly stent retrievers has recently been shown to be more effective than IV thrombolysis alone in well-selected patients with proximal intracranial occlusions of the anterior circulation18 and is now considered the standard of care.19,20 This is because recanalization is one of the most important predictors of prognosis in AIS,21,22 and proximal occlusions insufficiently respond to IV thrombolysis in many cases.23 Endovascular stroke trials have mostly excluded patients with posterior circulation strokes. Although large posterior circulation occlusions are also likely to benefit from endovascular treatment, this benefit has to first be proved by a randomized controlled trial such as the Basilar Artery International Cooperation Study trial.24
The proportion of 40.7% of such patients in this tertiary care setting imaged within 6 hours provides an estimate of the number of patients who could benefit from such interventions. When adding clinical and other radiologic criteria as well as contraindications, this number may be reduced by half in the real world, however.25 Still, improved prehospital identification and transport of patients with probable proximal intracranial occlusions26 are likely to increase the number of endovascularly treatable patients in comprehensive stroke centers.27
The spontaneous recanalization rate of proximal occlusions between 4.5 and 24 hours from symptom onset is approximately 9% based on the aforementioned proportions of patients with such occlusions. In contrast, early digital subtraction studies reported an estimated 17% spontaneous recanalization at 6–8 hours from stroke onset.28
Limitations are the retrospective, uncontrolled nature of the study with data from a single-center registry, which may not have a population representative of other settings for acute stroke care. We excluded a certain number of patients because acute CTA was not performed, mainly due to contrast contraindications. Images of head and neck vessels were analyzed in the arterial phase only, possibly overestimating the degree and extent of proximal occlusive disease. Also, the results are based on CTA, which is a reliable method for assessment of arterial pathology in AIS4,29,30 but may not be generalizable to other angiographic methods. We did not analyze associations among arterial imaging, clinical variables, and functional outcome because this was the purpose of recent publications.26,31
On the other hand, the available data may represent the best possible approximation of a real-world AIS population, given that some patients will always be unable to undergo acute angiographic evaluations.
Conclusions
This study gives a comprehensive picture of the frequency and distribution of relevant arterial pathology on CTA obtained within 24 hours of symptom onset. Approximately half of patients had relevant arterial pathology in the ischemic territory, mostly in the intracranial anterior circulation; a significant number also had tandem pathology. The 40% of patients with AIS having proximal intracranial arterial occlusions in the first 6 hours give an estimate of the eligibility for acute endovascular therapy.
Footnotes
David C. Rotzinger and Pascal J. Mosimann contributed equally to this work.
Disclosures: David Rotzinger—UNRELATED: Employment: Lausanne University Hospital (Centre Hospitalier Universitaire Vaudois), Comments: my employer since 2011. Patrik Michel—RELATED: Grant: Swiss Heart Foundation*; UNRELATED: Board Membership: Boehringer-Ingelheim, Bayer Healthcare, Pfizer*; Consultancy: Pierre-Fabre, AstraZeneca, Amgen*; Payment for Lectures Including Service on Speakers Bureaus: Boehringer-Ingelheim, Bayer Healthcare, Medtronic-Covidien, Stryker*; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Boehringer-Ingelheim, Bayer Healthcare.* *Money paid to the institution.
The Swiss Heart Foundation supported this project.
Paper previously presented at: The Joint annual meeting 2014 (Swiss Society of Intensive Care Medicine, 190th Annual Meeting of the Swiss Neurological Society, Swiss Society of Neuroradiology SSNR Educational Course 2014, Swiss Stroke Society, Swiss Society of Emergency Medicine), October 29–31, 2014; Congress Centre Kursaal Interlaken, Switzerland; and Annual Meeting of the Swiss Congress of Radiology, June 8–10, 2014; Davos, Switzerland.
Indicates open access to non-subscribers at www.ajnr.org
REFERENCES
- Received August 23, 2016.
- Accepted after revision December 13, 2016.
- © 2017 by American Journal of Neuroradiology