Abstract
BACKGROUND AND PURPOSE: The causative gene of the common congenital malformation referred to as CHARGE syndrome is CHD7. Affected individuals often undergo head and neck imaging to assess abnormalities of the olfactory structures, hypothalamus-pituitary axis, and inner ear. We encountered a few children with severe hypoplasia of the basiocciput during a radiologic assessment of patients with CHARGE syndrome. To our knowledge, this anomaly has not been reported. Our purpose was to evaluate the incidence and severity of this anomaly in this syndrome.
MATERIALS AND METHODS: Sagittal MR images of 8 patients with CHARGE syndrome were retrospectively reviewed by 2 radiologists who consensually evaluated the status of the basiocciput of the patients with CHARGE syndrome, as either normal or hypoplastic; and associated anomalies, which include basilar invagination, Chiari type I malformation, and syringomyelia, as either present or absent. The length between the basion (Ba) and the endo-sphenobasion (Es) and between the basion and the exo-sphenobasion (Xs) was measured on midsagittal MR images of the 8 patients and 70 age-matched controls. We searched for trends related to age in the length of Ba-Es and Ba-Xs of the control children by using a matched t test.
RESULTS: Basioccipital hypoplasia was identified in 7 of the 8 patients with CHARGE syndrome and was severe in 6. Of those, 5 had associated basilar invagination and 1 had Chiari type I malformation with syringomyelia.
CONCLUSIONS: Basioccipital hypoplasia and basilar invagination are prevalent in patients with CHARGE syndrome.
CHARGE syndrome is a widespread malformation that was originally described independently by Hall 1 and Hittner et al.2 The mnemonic acronym CHARGE represents the major anomalies associated with the disorder: coloboma of the eye, heart defects, choanal atresia, retarded growth and development, genital hypoplasia, and ear anomalies.3 Other cardinal features include facial palsy or facial asymmetry, anomalies of the inner ear and laryngotracheoesophagus, anosmia, hypogonadotropic hypogonadism, and orofacial clefts.3-6 The phenotypic diversity of affected individuals has raised the notion that CHARGE is not a genuine syndrome but an association of various anomalies occurring in a nonrandom but inconsistent fashion. However, this hypothesis has been disputed by the recent discovery of a CHARGE syndrome gene, CHD7, which encodes the chromodomain helicase deoxyribonucleic acid (DNA)-binding protein 7.7 Nevertheless, locus heterogeneity of CHARGE syndrome might exist because only 60%–70% of patients with CHARGE syndrome have CHD7 mutations.8,9 Therefore, a more discriminating syndromic delineation and further studies of the genotype-phenotype correlation are required to understand this syndrome thoroughly.
Malformations of the inner ear, olfactory structures, and hypothalamus/pituitary axis in patients with CHARGE syndrome have usually been evaluated by using imaging techniques.10-12 We encountered a few children with severe hypoplasia of the basiocciput during a radiologic assessment of patients with CHARGE syndrome. Basiocciput hypoplasia results in shortening of the clivus and is always associated with basilar invagination.13 There is an increased prevalence of neural dysgenesis, such as the Chiari malformation or syringohydromyelia, reported to occur in 25%–35% of patients with basilar invagination.14 Here, we evaluated the incidence and severity of basioccipital hypoplasia in CHARGE syndrome.
Materials and Methods
Patients with CHARGE Syndrome
We retrospectively reviewed 8 patients who were diagnosed with CHARGE syndrome according to the clinical criteria of Blake et al (Table 1),15 in which patients with all 4 major criteria or with 3 major and 3 minor criteria are considered to have definitive CHARGE syndrome, whereas those with 1 or 2 major criteria and several minor criteria possibly have the syndrome. Five of our patients had definitive and 3 had possible CHARGE syndrome. Patients 3 and 5 fulfilled 2 major and 5 minor criteria, and patient 8 fulfilled 2 major and 3 minor criteria. Our institutional review board did not require its approval or informed consent for the retrospective evaluation of patients’ records and images. High-performance liquid chromatography DNA screening16 and direct sequencing proved that 7 of the 8 patients harbored heterozygous mutations in CHD7 (Table 2).
Diagnostic criteria of CHARGE syndrome according to Blake et al15
Characteristics and basioccipital findings of patients with CHARGE syndrome
Controls
Seventy age-matched controls comprising 10 individuals per group, 3, 6, 7, 8, 9, 11, and 21 years of age, were randomly selected from patients with normal findings on MR imaging at our hospital between 2006 and 2008. Patients with known or suggested abnormalities involving the skull base or bone marrow were excluded. Patients with systemic disease or who had undergone previous radiation or chemotherapy were also excluded.
Image Analysis of the Basiocciput
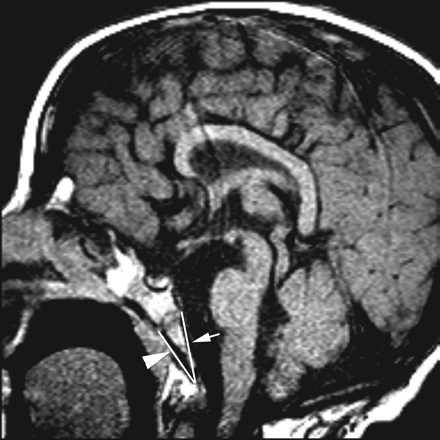
Sagittal MR images were obtained from all patients and all control children by using a 1.5T scanner. The images were reviewed on a PACS workstation. The length between the basion (Ba) and the endo-sphenobasion (Ba-Es) and between the Ba and the exo-sphenobasion (Ba-Xs) was measured in accordance with the definitions of Ba, Es, and Xs on midsagittal images (Fig 1). The definitions of Ba, Es, and Xs are the midpoint on the anterior margin of the foramen magnum, the anteriormost midpoint on the dorsal aspect of the basiocciput, and the anteriormost midpoint on the ventral aspect of the basiocciput, respectively. The midsagittal image is defined as that on which the clivus, pituitary infundibulum, and aqueduct of the cerebrum are delineated in a single plane. Measurements were obtained from 2- or 3-mm-thick sections of T1-weighted midsagittal spin-echo (SE) images (TR/TE/NEX, 400–500 ms/10–15 ms/2) from patients with CHARGE syndrome. Control measurements were obtained from 4-mm-thick sections of T2-weighted midsagittal half-Fourier acquired single-shot turbo spin-echo images (TR/TE/flip angle, 1500 ms/101 ms /170°). Each measurement was obtained 5 times by 1 radiologist (K.F.).
Measurement of the lengths of Ba-Es (arrow) and Ba-Xs (arrowhead) on a T1-weighted sagittal image (patient 3).
Evaluation of the presence or absence of basioccipital hypoplasia, basilar invagination, Chiari type I malformation, and syringomyelia among the patients with CHARGE syndrome was performed by using 2- or 3-mm-thick sections of T1-weighted SE sagittal images (TR/TE/NEX, 400–500 ms/10–15 ms/2) in all patients, T2-weighted fast SE sagittal images (TR/TE/NEX, 2000–3000 ms/92–94 ms/1) in all the patients except patient 1, and 4-mm-thick sections of T1-weighted fast SE sagittal images (TR/TE/NEX, 337 ms/12 ms/1) and T2-weighted fast SE images (TR/TE/NEX, 1600 ms/170 ms/1) of the whole spine of patient 4. The MR images of the patients were consensually reviewed by 2 board-certified radiologists (K.F. and N.A.).
Basioccipital hypoplasia was defined as follows: Mild hypoplasia defined in patients in whom the length of either the Ba-Es or the Ba-Xs was <2 SDs compared with the normal values for each age group and with a maintained triangular shape. Moderate hypoplasia was defined as patients in whom the length of either Ba-Es or Ba-Xs was <2 SDs and without maintenance of a triangular shape; and severe hypoplasia was defined as patients in whom the lengths of both Ba-Es and Ba-Xs were <2 SDs and without maintenance of a triangular shape. The basilar invagination was when the tip of the odontoid lay 5 mm above the Chamberlain line, which joins the posterior margin of the foramen magnum to the posterior margin of the hard palate. Herniation of at least 1 cerebellar tonsil ≥5 mm below the foramen magnum was considered to be Chiari type I malformation. A cavity and/or a dilated central canal of the spinal cord was considered to be the syringomyelia.
Statistical analysis was performed by using a matched t test to search for trends related to age in the length of Ba-Es and Ba-Xs of the control children. A P value of < .05 was considered to represent a statistically significant difference.
Results
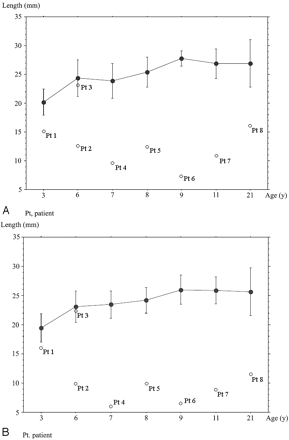
Table 3 and Fig 2 show the lengths of the Ba-Es and Ba-Xs in the controls. The lengths of Ba-Es and Ba-Xs in the control individuals of the 3-year-old group were significantly shorter than those at other ages (P = .023, P = .0015). The Ba-Es was significantly elongated in the 8- and 9-year-olds (P = .0479), but none of the other values significantly differed among the age groups.
Line graph and bars show means and SDs of the length of Ba-Es (A) or Ba-Xs (B) for each age group of healthy controls. White dots show the length of Ba-Es or Ba-Xs of patients with CHARGE syndrome. Pt indicates patient.
Lengths of Ba-Es and Ba-Xs in control individuals at various ages
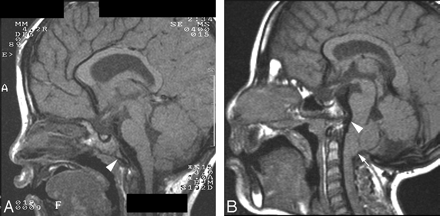
Table 2 and Fig 2 summarize the Ba-Es and Ba-Xs lengths and imaging findings from patients with CHARGE syndrome. The basiocciput was hypoplastic in 7 of the 8 patients (6/7 patients with CHD7 mutations and patient 8 who did not undergo molecular analysis) and was accompanied by a shorter Ba-Es and Ba-Xs. Basioccipital hypoplasia was severe in 6 and mild in 1 patient (Fig 3A). Basilar invagination was identified in 5 of 6 patients with severe basioccipital hypoplasia (Fig 3B). One of the 5 patients had a Chiari type I malformation with syringomyelia that involved the lower cervical and whole thoracic spinal cord. This patient also had atrophy of the hands.
T1-weighted sagittal images of patients 1 (A) and 4 (B). A, Mild hypoplastic basiocciput (arrowhead). B, Severe hypoplastic basiocciput (arrowhead) with basilar invagination and Chiari type I malformation (arrow).
Four of the 8 patients had orofacial clefts, and 3 of these 4 had severe basioccipital hypoplasia.
Discussion
The anatomy of the clival region has been described from various morphologic or clinical viewpoints. Lang and Issing17 (cited by Krompotić-Nemanić et al18) reported that the width and length of the adult clivus is about 28 mm and from 52 to 54 mm, respectively, and Krompotić-Nemanić et al reported postnatal changes in the dimensions of the clivus.18 However, postnatal changes in the length of the basiocciput, a component of the clivus, have not been reported as far as we can determine. Our study of control individuals ranging from 3 to 21 years of age showed that the occiput became significantly elongated between 3 and 6 years of age and again between 8 and 9 years of age, but not after 10 years of age. Our findings are compatible with anatomic findings showing that the length of the clivus is maximal before puberty and the second growth spurt.18
The present study showed that basioccipital hypoplasia and basilar invagination are prevalent in CHARGE syndrome. However, our series was biased, and we analyzed only a small number of patients with this syndrome because patients with CHARGE syndrome and CHD7 mutations were more likely to undergo imaging.19 Thus, additional studies of the incidence and severity of CHARGE syndrome without CHD7 mutations are mandatory; however, we tentatively concluded that routine assessment of the basiocciput in patients with CHARGE syndrome is helpful to exclude potentially life-threatening basilar invagination regardless of the presence or absence of CHD7 mutations.
CHARGE syndrome accompanied by basilar invagination is classified as the primary type as a result of a hypoplastic basiocciput, in contrast to the secondary type that is associated with soft bone conditions, such as hyperparathyroidism, osteomalacia, and Paget disease. Primary basilar invagination results from a congenital anomaly of the chondrocranium.20,21 The malformed clivus and/or translocated odontoid peg impinges on the anterior craniospinal neuroaxis and causes neurologic symptoms, including upper motor neuron deficits, cranial nerve abnormalities, hydrocephalus, cerebellar dysfunction, syringomyelia, and even sudden death.22-24 One of the patients with basilar invagination had Chiari type I malformation with syringomyelia and developed neurologic symptoms. Symptomatic patients will require surgical procedures for relief of symptoms because the ability to reduce basilar invagination is age-related.25
The pathogenesis of the hypoplastic basiocciput in CHARGE syndrome remains elusive. The sole known causative gene (CHD7) for the syndrome encodes chromodomain helicase DNA-binding protein 7 (CHD7 protein), which belongs to the chromodomain family. In general, chromodomain family proteins are involved in the maintenance of chromatin structures and are expressed in mesenchymal cells derived from the neural crest. CHD7 protein is mainly expressed in epithelial cells, olfactory epithelium, and eye, ear, and kidney tissues as well as the vascular system.26 During the early embryonic stage, CHD7 protein is preferentially expressed in the undifferentiated neuroepithelium and mesenchyme of neural crest origin.27 These facts closely correspond with the clinical manifestations of CHARGE syndrome.
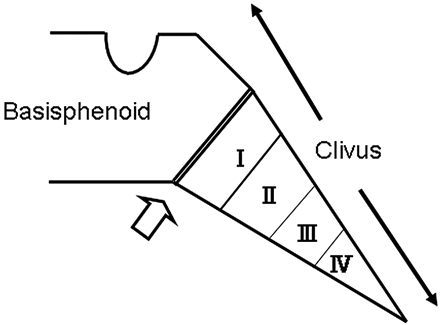
However, the basiocciput, unlike the facial bones, derives from the mesodermal cells of the occipital somites and not from the neural crest (Fig 4).13,28 Thus far, the CHD7 protein has not been identified in the occipital somites. To understand the association between CHD7 mutations and the hypoplastic basiocciput, one must assume interaction between the neural crest and somite cells during development. Cleft lip and palate, which are thought to result from impaired neural crest cells, are associated with shortening of the clivus.29 In addition, deviation of the cranial base in dimension and shape is described in complete cleft lip and palate.29-31 These facts might support the notion of a developmental link between the facial bones and the basiocciput. However, orofacial clefts and hypoplastic basiocciput did not closely correspond in our series; only 3 of our 8 patients with severe basioccipital hypoplasia had orofacial clefts, whereas 1 without basioccipital hypoplasia had the anomaly. Thus, the relationship between basioccipital hypoplasia and maldevelopment of neural crest cells in CHARGE syndrome remains elusive.
Contribution of the basiocciput, formed by 4 occipital sclerotomes (I-IV), to the lower portion of clivus. The upper potion is formed by the basisphenoid. The spheno-occipital synchondrosis (open arrow) lies in between.
With increasing experience, an expert group of geneticists and developmental pediatricians defined the major and minor criteria of CHARGE syndrome.15 In the radiologic diagnostic methods, Chalouhi et al11 reported that anomalies of the olfactory bulbs and tracts might be pathognomonic for CHARGE syndrome and should be included as a major criterion for the diagnosis of this syndrome; Blustajn et al10 recently reported that these anomalies were the most prevalent features of CHARGE syndrome. The present study showed that basioccipital hypoplasia and basilar invagination are significantly prevalent in the syndrome. If our data are confirmed in a larger group of patients with CHARGE syndrome, these anomalies could serve as new criteria for the diagnosis. MR imaging enables simultaneous assessment of the semicircular canal, olfactory structures, and the basiocciput; and simultaneous detection of abnormalities involving these structures might enable us to make the imaging diagnosis of CHARGE syndrome.
Although the number of patients with this syndrome in the present study was small, basiocciput hypoplasia and basilar invagination were prevalent in our patients.
Conclusions
Basioccipital hypoplasia and basilar invagination are prevalent in patients with CHARGE syndrome. Routine assessment of the basiocciput in patients with CHARGE syndrome is helpful to exclude potentially life-threatening basilar invagination; identification of these anomalies might help to accurately diagnose CHARGE syndrome.
References
- Received August 6, 2008.
- Accepted after revision September 25, 2008.
- Copyright © American Society of Neuroradiology