Abstract
BACKGROUND AND PURPOSE: White matter signal-intensity abnormalities (WMSA) on MR imaging are related to adverse neurodevelopmental outcome in extremely preterm infants. Diffusion tensor imaging (DTI) may detect alterations in cerebral white matter microstructure and thus may help confirm the pathologic basis of WMSA. This study aimed to relate regional DTI measures with severity of WMSA in very preterm infants.
MATERIALS AND METHODS: One hundred eleven preterm infants (birth weight, <1250 g and/or gestational age, <30 weeks) were scanned at term-equivalent age (1.5T). WMSA were classified as normal, focal, or extensive. Apparent diffusion coefficient (ADC), fractional anisotropy (FA), axial (λ1), and radial ([λ2 + λ3]/2) diffusivity were calculated in 12 regions of interest placed in the bilateral posterior limbs of the internal capsule, frontal (superior and inferior), sensorimotor, and occipital (superior and inferior) white matter regions. Data were compared by using 1-way analysis of variance, with a Bonferroni correction for multiple comparisons.
RESULTS: Thirty-nine infants had normal, 59 infants had focal, and 13 infants had extensive WMSA. Compared with infants with normal or focal WMSA, infants with extensive WMSA had significantly lower FA in the internal capsule (P < .001), right inferior frontal regions (P < .05), and right superior occipital regions (P = .01); and higher radial diffusivity in the right internal capsule (P = .005), bilateral sensorimotor (P < .05), and right superior occipital regions (P < .05). Compared with infants with normal WMSA, infants with extensive WMSA had significantly higher ADC in bilateral sensorimotor regions (P < .01) and right superior occipital regions (P = .01), and lower axial diffusivity in the bilateral sensorimotor regions (P < .05).
CONCLUSIONS: There are significant region-specific changes in ADC, FA, radial diffusivity, and axial diffusivity in preterm infants with extensive WMSA. Altered radial diffusivity was most prominent. This implies that disrupted premyelinating oligodendroglia is the major correlate with extensive WMSA rather than axonal pathology.
Extremely preterm infants are at high risk of brain injury and subsequent cognitive and motor disability.1,2 The main cerebral pathologies in the preterm neonate are germinal matrix-intraventricular hemorrhage, posthemorrhagic hydrocephalus, and periventricular leukomalacia (PVL). Cystic PVL is readily identified by cranial ultrasonography and is well recognized as being an important predictor of motor disability. However, with the availability of MR imaging, it is now increasingly evident that noncystic PVL is the most common white matter abnormality in the preterm infant brain.3,,4 The 2 main appearances on MR imaging of noncystic white matter injury in ex-preterm infants have been described as “diffuse excessive high signal intensity” (DEHSI) on T2-weighted images and focal or multifocal shortening of T1 and T2 relaxation times in the white matter.4-6 These classifications are based on qualitative assessments and have been criticized because these “abnormalities” may represent normal developmental change or imaging artifacts.7 However, these white matter signal-intensity abnormalities (WMSA) have been demonstrated to be important predictors of subsequent motor dysfunction and poor developmental outcome.6,8,9
Diffusion tensor MR imaging (DTI) assesses random motion of water molecules within biologic tissue and can provide insight into white matter microstructure and development. With DTI, several measures of water diffusivity, including apparent diffusion coefficient (ADC), fractional anisotropy (FA), and radial and axial diffusivity, can be obtained. ADC is a measure of water diffusion within tissue and is influenced by random thermal motion and the presence of cell membranes, macromolecules, and white matter fibers.10 Water displacement within tissue can be represented by 3D diffusion ellipsoids, which reflect 3 major eigenvectors, λ1, λ1, and λ3. The degree to which the diffusion ellipsoid deviates from being spheric is quantitated by FA values, which are zero for isotropic diffusion and increased for higher degrees of anisotropy. Mathematically, the FA is the SD of the eigenvalues of the diffusion tensor divided by the ADC and is given as a percentage. The primary eigenvector (λ1) corresponding to the maximum diffusivity is oriented parallel to white matter fibers. Its eigenvalue is referred to as “axial diffusivity,” where higher values reflect better axonal integrity. The secondary (λ2) and tertiary (λ3) eigenvectors are assumed to be in the transverse plane oriented perpendicular to the fiber direction. The average of their eigenvalues is referred to as “radial diffusivity,” and higher values indicate that more water diffusion is occurring perpendicular to axons, representing less myelination or reduced oligodendroglial integrity.
The value of diffusion MR imaging in the preterm infant has been under much investigation. Diffusion abnormalities have been shown to be present before qualitative abnormalities in the white matter, including signal-intensity changes and cystic formation in the unmyelinated neonate brain.11-13 Indeed in preterm infants with white matter abnormalities on MR imaging, diffusion parameters are altered as well.14-16 The classification of the white matter abnormalities in those studies differed, ranging from DEHSI to overt white matter lesions on MR imaging. In addition, these studies were based on a relatively small numbers of infants. Thus, there is a need to further establish in a larger cohort that WMSA on T1- and T2-weighted MR imaging in preterm infants are a reflection of underlying pathology rather than imaging artifacts alone.
The aim of this study was to compare the diffusion parameters (ie, ADC, FA, axial diffusivity, and radial diffusivity) from several preassigned cerebral white matter regions with the severity of WMSA graded on conventional T1- and T2-weighted MR imaging in a large cohort of extremely preterm infants.
Materials and Methods
Patients
This study is part of a large cohort study assessing preterm brain development by using MR imaging. Two hundred twenty-seven preterm infants (birth weight, <1250 g or gestational age, <30 weeks) were recruited during a 3-year period at the Royal Women's Hospital in Melbourne. Permission was granted by the Royal Women's Hospital Research and Ethics Committees, and written informed parental consent was necessary for participation in this study.
MR Imaging
MR imaging examination was performed between 38 and 42 weeks’ corrected gestational age by using a 1.5T Signa LX Echospeed system (GE Healthcare, Milwaukee, Wis). We performed the following sequences: 1) a 3D Fourier transform spoiled gradient recalled-echo sequence (1.5-mm coronal sections; flip angle, 45°; TR, 35 ms; TE, 5 ms; FOV, 18 cm; matrix, 256 × 256); 2) a double-echo (proton-attenuation and T2-weighted) spin-echo sequence (3-mm axial sections; TR, 3000 ms; TE, 36 and 162 ms; FOV, 18 cm; matrix, 256 × 256, interleaved acquisition); and 3) a line scan sequence (4- to 6-mm axial sections with a 0.5- to 1-mm gap; TR, 2139 ms; TE, 78 ms; FOV, 22 cm; matrix, 128 × 128; 2 images at b=5 s/mm2; 6 images at b=700 s/mm2. The diffusion gradients for b=700 s/mm2 were oriented in 6 noncollinear directions). Before the MR imaging examination, infants were fed, swaddled, and placed in a Vac Fix beanbag (S&S Par Scientific, Odense, Denmark) designed to keep the infant still and supported in the MR imaging scanner.
MR images were qualitatively classified by using a standardized system3,5 by 2 independent raters who were blinded to the clinical history and cranial sonographic findings. The MR imaging diagnosis of WMSA required either T1 hyperintensity in the absence of marked T2 hypointensity (which was interpreted as astrogliosis) or T1 hyperintensity with substantial T2 hypointensity (which was interpreted as foci of hemorrhage) or significant T2 hyperintensity (excessive hyperintensity). The classification into “focal” and “extensive” was a reflection of how widespread these WMSA were present in the neonate brain. MR images were classified as having a “focal” WMSA if 1 lobar region was involved, and “extensive” WMSA if >1 lobar region was involved or cystic change indicative of areas of tissue dissolution was present.
DTI Analysis
For each voxel, 3 eigenvalues and eigenvectors of the diffusion tensor and the ADC were calculated. Apparent diffusion was measured according to the Stejskal and Tanner equation,17 with the diffusion tensor elements estimated for each voxel by nonlinear regression, as suggested by Basser and Pierpaoli.18
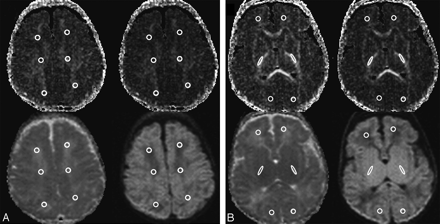
DTI analysis was performed off-line by using XPhase image analysis software (SE Maier, Boston, Mass). ADC, FA, axial (λ1), and radial (λ2 + λ3)/2 diffusivity were calculated in 12 manually selected regions of interest in the brain, located on 2 axial sections. The region-of-interest placement was performed by 1 individual and then checked by another who was experienced in neonatal neuroimaging. The superior axial section was taken at a level just above the lateral ventricles, and regions of interest were located in bilateral frontal, sensorimotor, and occipital white matter regions (Fig 1A). The inferior section was taken at a level through the basal ganglia and posterior limb of the internal capsule (PLIC), and regions of interest were located in the bilateral middle third of the PLICs and the frontal and occipital white matter regions (Fig 1B). The diffusion-weighted, ADC, relative anisotropy, and FA images were viewed simultaneously in the axial plane for region-of-interest placement.
A, Axial line scan images obtained at the level just above the superior margins of the lateral ventricles. The 4 images used to determine the regions of interest were the following: 1) FA (top left), 2) relative anisotropy (top right), 3) ADC (bottom left), and 4) diffusion-weighted (bottom right). Six regions of interest were located in the following regions: frontal white matter, sensorimotor white matter, and occipital white matter. B, Axial line scan image obtained at the level of the basal ganglia and PLIC. The 4 images used to determine the regions of interest were the following: 1) FA (top left), 2) relative anisotropy (top right), 3) ADC (bottom left), and 4) diffusion-weighted (bottom right). Six regions of interest were located in the following regions: frontal white matter, middle third of the PLICs, and occipital white matter.
Statistical Analysis
Diffusion measures from the 3 groups of varying severity of white matter abnormalities on MR imaging were compared by using 1-way analysis of variance, with a Bonferroni correction for multiple comparisons. A P value < .05 (following Bonferroni correction) was considered significant.
Results
Of the 227 preterm infants who were recruited, 111 had MR imaging examinations at term-corrected age in which all 3 sequences (ie, conventional T1-, T2-weighted, and diffusion-weighted line scan) were obtained and the data were analyzable. The clinical characteristics of the infants are summarized in Table 1. There were no significant differences in any of the clinical characteristics between the subgroup analyzed for this study and the whole preterm cohort (data not shown).
Clinical characteristics of infants
ADC in Relation to WMSA
Compared with infants with normal WMSA, infants with extensive WMSA had significantly higher ADC in the right superior occipital and bilateral sensorimotor regions (Table 2).
Relationship between ADC (10−3 mm2/s) and WMSA*
FA in Relation to WMSA
Infants with extensive WMSA had significantly lower FA in bilateral internal capsules and right frontal and right occipital regions compared with infants with normal and focal WMSA on MR imaging (Table 3).
Relationship between FA (%) and WMSA*
Axial Diffusivity in Relation to WMSA
Compared with infants with normal WMSA, infants with extensive WMSA had significantly lower axial diffusivity in the right internal capsule and significantly higher axial diffusivity in bilateral sensorimotor regions (Table 4).
Relationship between axial diffusivity (×10−3 mm2/s) and WMSA*
Radial Diffusivity in Relation to WMSA
Compared with infants with normal WMSA, infants with extensive WMSA had significantly higher radial diffusivity in the right inferior frontal, right internal capsule, and right superior occipital and bilateral sensorimotor regions (Table 5). In addition, compared with infants with focal WMSA, infants with extensive WMSA had significantly higher radial diffusivity in the regions listed above, with the exception of the right superior frontal region.
Relationship between radial diffusivity (×10−3 mm2/s) and WMSA*
In all occasions, there was a trend for stepwise reductions in the anisotropy and increases in the ADC across the grading of severity of brain injury.
Discussion
This study has demonstrated important associations between a global WMSA score on MR imaging and diffusion parameters. Increased radial diffusivity and decreased FA in the internal capsules and sensorimotor regions of infants with extensive WMSA on MR imaging were the most prominent findings. In addition, ADC was increased in the right superior occipital and bilateral sensorimotor regions, and axial diffusivity was decreased in the right internal capsule and bilateral sensorimotor regions. These findings suggest that extensive WMSA on conventional T1- and T2-weighted MR imaging are associated with important microstructural abnormalities, in particular, radial diffusivity. This implies that the major correlate of extensive WMSA on MR imaging may be the disruption within the premyelinating oligodendroglia rather than axonal pathology. In addition, the results also suggest that there are vulnerable brain regions that display more microstructural derangement on diffusion imaging, including the sensorimotor region and internal capsules.
Altered diffusion parameters have been documented in preterm infants with abnormal white matter on MR imaging and in those with abnormal neurologic outcome. Previous studies have found that mean ADC increased in preterm infants with white matter abnormalities on MR imaging, including diffuse excessive high-signal intensities on MR imaging, and in infants with overt white matter lesions (eg, diffuse or nodular white matter hypo- or hyperintensities and cystic lesions).14,15 Relative anisotropy was lower in infants with white matter lesions, and it was speculated that this may have been due to a decrease in directionality as a result of fiber tracts that were disrupted. Subsequent to these studies, radial diffusivity and axial diffusivity were also found to be deranged in infants with diffuse excessive WMSA on MR imaging.16 The authors concluded that the findings may have reflected delayed myelination in infants with white matter abnormalities on MR imaging.
The findings from this study are in keeping with previously published studies. We found abnormal diffusion parameters, in particular radial diffusivity in infants with extensive WMSA on MR imaging. Radial diffusivity describes water ADC perpendicular to axons. Data from studies on mature animals have shown that primary injury to myelin is associated with an increase in radial diffusivity, whereas primary injury to axons, such as wallerian degeneration, is associated with a decrease in axial diffusivity.19,20 However, our studies are at a stage of brain development in which there is little myelination but an abundance of premyelinating oligodendrocytes in the human cerebral white matter. During this maturational phase, the early differentiating oligodendrocytes are beginning to ensheathe cerebral white matter axons, in preparation for myelination, which happens after term in the human.21-23 These changes in the oligodendroglia, which subsequently result in changes in axonal size, axonal membranes, and intracellular axonal constituents, are likely to contribute to the normal decreases in the extracellular space and water content in cerebral white matter and thus to the normal decline in ADC and increases in FA and radial anisotropy.24-26 Thus, 1 hypothesis to explain the changes demonstrated in this study and previous studies for the failure of the reduction in ADC from higher levels in the premature infant to the lower levels of the term infant in the presence of WMSA on MR imaging may relate to prior injury to premyelinating oligodendrocytes and the subsequent failure of their development and ensheathment of axons.27
In addition, there were inconsistent findings in relation to axial diffusivity, with a reduction in the internal capsule but an increase in the sensorimotor cortex regions. The internal capsule is a very tightly packed cabling network; thus, it is plausible that there may be a lack of sensitivity for the measures in these densely packed fiber tracts. The infants in this study were imaged at term-corrected age, many weeks after birth and presumed time of insult. Thus, chronic alterations in axial diffusivity consistent with axonal loss may be more visible in the less densely packed fibers as they emerge and spread into the sensorimotor region.
There were no significant differences in any of the diffusion parameters in infants with normal and focal WMSA. This would suggest that less extensive WMSA changes on conventional T1- and T2-weighted MR imaging in ex-preterm infants were not associated with significant MR imaging−detectable microstructural disruption on these diffusion analyses during this period of brain development. There are, however, limitations in the diffusion data that were acquired in this study that may have led to a reduction in sensitivity to milder forms of microstructural disruption. The data were obtained at a low field strength (1.5T) with a large section thickness (4–6 mm) and a very limited number of 6 directions. Newer diffusion imaging techniques with more directions and higher resolution in higher field magnets may have greater sensitivity for milder forms of white matter microstructural disturbance.
Another notable finding from this study relates to the fact that altered diffusivity was region-specific, involving particularly the sensorimotor pathways of the brain. Severe white matter abnormality on MR imaging is known to be most strongly associated with motor dysfunction.6 We found that many of the diffusion alterations involved the sensorimotor regions and the internal capsules, which are areas of increased white matter maturity in the neonate. This may partly explain the strong relationship between qualitative extensive white matter abnormalities in ex-preterm infants and poor neuromotor outcome.
In comparison with previous studies, which have been on smaller cohorts (<50 infants), our findings have been based on a much larger cohort. The consistent finding that abnormal diffusion parameters are associated with WMSA further adds weight to existing evidence that WMSA on MR imaging are related to abnormal tissue microstructure and are not solely explained by image artifacts or windowing.
One of the drawbacks of the current study is that the diffusion information was obtained from prespecified regions of interest within the brain. It would be useful to obtain diffusion information from “diffusion maps” encompassing the whole brain to be able to further define the abnormal areas within the brain in ex-preterm infants with WMSA. With technical advancements in postacquisition diffusion analysis, it may be possible to derive diffusion maps from similar cohorts in the future.
Future directions in this research area would also include MR tractography to explore the associations between altered diffusivity and altered fiber tract development in these infants. The infants in this study are being longitudinally evaluated for neurodevelopmental outcomes; thus, evaluation of the relationships between diffusion measures, WMSA, and childhood outcomes will be possible.
Conclusions
Signal-intensity abnormalities in the white matter on MR imaging in ex-preterm infants at term-corrected age are associated with derangement in all diffusion parameters, with radial diffusivity being the most prominent. The severity of the derangement is related to the severity of the regional involvement of WMSA. These findings in vivo concur with in vitro and experimental studies that imply disruption of premyelinating oligodendroglia as the major correlate with extensive WMSA in the preterm neonate.
Acknowledgments
We thank Merilyn Bear for her assistance with recruitment, liaison with families, and care of babies in the MR imaging scanner and all the families for their willingness to participate in this study.
Footnotes
This work was supported by grants from the National Health and Medical Research Council of Australia (project grant number 237117), Murdoch Children's Research Institute, The Royal Women's Hospital Research Foundation, and the Jack Brockhoff Foundation.
References
- Received August 19, 2008.
- Accepted after revision October 5, 2008.
- Copyright © American Society of Neuroradiology