Abstract
BACKGROUND AND PURPOSE: Corpus callosum lesions are of specific interest in the evaluation of suspected multiple sclerosis in brain MR imaging. Using thin-section sagittal fluid-attenuated inversion recovery images, researchers have shown that the finding of “subcallosal striations” correlates significantly with the diagnosis of multiple sclerosis. Using the same MR imaging technique, we describe a finding of ependymal irregularity that we call the “Dot-Dash” sign, which we believe to be associated with early multiple sclerosis.
METHODS: Sagittal 2-mm fast fluid-attenuated inversion recovery images were obtained in 70 patients. Thirty-five patients had multiple sclerosis according to the Poser criteria, and 35 were age-matched controls. The images were reviewed in a blinded fashion by an experienced neuroradiologist for the presence or absence of the Dot-Dash sign.
RESULTS: The correlation between the Dot-Dash sign and definite clinical multiple sclerosis is highly significant (P < .001), with a sensitivity of 91.4% and a specificity of 65.7%. In the age group of ≤50 years, the sensitivity was 95.7% and the specificity, 71.9%.
CONCLUSION: The Dot-Dash sign of ependymal irregularity on thin-section sagittal fluid-attenuated inversion recovery images is an early marker for multiple sclerosis, which is particularly useful in the younger patient. This finding appears to be more sensitive for early lesion detection than any other multiple sclerosis imaging finding yet described in the literature.
MR imaging is the primary technique for identifying white matter lesions caused by multiple sclerosis (1–4).
The development of advanced MR imaging has increased the sensitivity and specificity for lesion detection and characterization during the past 20 years. T2-weighted fast fluid-attenuated inversion recovery sequences show the greatest sensitivity for lesion detection because of increased contrast, especially in the subependymal and periventricular regions (5–7). Although new MR imaging techniques like diffusion tensor imaging have recently been described (8), they are not widely available. Thus fluid-attenuated inversion recovery imaging is still the key MR imaging sequence for multiple sclerosis.
Various pathognomonic patterns of disease distribution for multiple sclerosis have been described in the literature (9), involving the periventricular white matter (10) and the corpus callosum (11). Dawson finger (perivenular collections of inflammatory cells) (12) was described in the pathology literature more than 100 years ago. It corresponds to the “ovoid” lesions on MR imaging (13) and the smaller “subcallosal striations” (5, 14). These signs have particular utility because of relatively high sensitivity and specificity when compared with more nonspecific appearances of other white matter lesions on MR imaging.
Herein we describe an MR imaging finding that is also highly sensitive for multiple sclerosis—an ependymal irregularity that we call the “Dot-Dash” sign. This sign appears to suggest a diagnosis of multiple sclerosis at an earlier stage than other previously described white matter findings. The purpose of this article is to prospectively evaluate the sensitivity and specificity of the Dot-Dash sign for detection of multiple sclerosis.
Methods
Patients
We prospectively evaluated 35 consecutive patients (mean [±SD] age, 44.4 ± 9.1 years; range, 16–77 years; 8 men; 27 women) who had a diagnosis of clinically definite multiple sclerosis based on clinical follow-up, using the Poser criteria (15). Follow-up was obtained by contacting the referring physician. Patients who did not fulfill the Poser criteria were not included in the study. One of the purposes of the clinical follow-up was to exclude patients with acute disseminated encephalomyelitis. The control population consisted of 35 consecutive patients (mean age, 38.5 ± 8.3 years; range, 12–64 years; 10 men; 25 women) who had undergone MR imaging of the brain ordered for a reason other than suspected demyelinating disease (history of migraine/headache, 6; suspected or known brain/spinal cord tumor, 2; nonspecific neurologic symptoms, 8; follow-up of nonspecific white-matter disease, 2; ophthalmopathy, 6; hearing loss, 1; infectious disease, 1; neurofibromatosis, 2; seizure, 2; vertigo, 1; other, 4). All patients gave permission for their medical information and images to be used in a confidential manner as approved by the local institutional review board.
MR Imaging
All scanning was performed by using a 1.5-T MR scanner (Signa Horizon EchoSpeed, GE Healthcare, Waukesha, WI or Magnetom Vision, Siemens Medical Systems, Erlangen, Germany). Two-millimeter-thick sagittal fast fluid-attenuated inversion recovery images (2D fast spin-echo inversion recovery: TR/TE/TI, 8800/130/2200; 1 mm gap; 256 × 192 matrix; 22-cm field of view) were used for this study and added to the standard brain MR imaging protocol (sagittal and axial T1-weighted conventional spin-echo, axial dual-echo PD/T2 CSE, and fast spin-echo fluid-attenuated inversion recovery. The thin-section sagittal fluid-attenuated inversion recovery images covered the corpus callosum and the periventricular white matter between the lateral aspect of the temporal horns bilaterally.
Definition of the Dot-Dash Sign
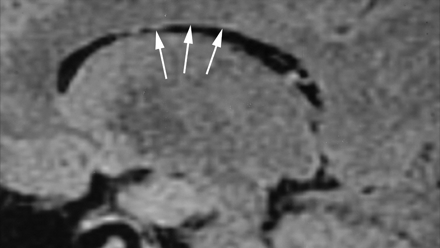
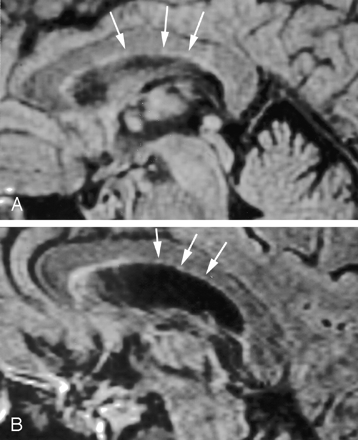
On thin-section sagittal fluid-attenuated inversion recovery images of the normal brain, there is a 1-mm thick hyperintense ependymal stripe (Fig 1). The Dot-Dash sign can be visualized as an irregularity of this ependymal stripe on the undersurface of the corpus callosum. We define it as at least 2 “dots” connected by a “dash” (Figs 2 and 3). On fluid-attenuated inversion recovery images, the “dot” is a round hyperintense irregularity of the ependymal undersurface with a diameter larger than the thickness of the “dash” adjacent to it. The “dash” is the remaining normal ependymal stripe. The Dot-Dash sign is not oriented perpendicular to the ependyma; this difference helps in distinguishing it from other multiple sclerosis signs such as subcallosal striations or ovoid lesions.
Sagittal fluid-attenuated inversion recovery image (TR/TE/TI, 8800/130/2200) shows a normal smooth ependymal stripe (arrows) without irregularity, subcallosal striations, or Dot-Dash sign.
Sagittal fluid-attenuated inversion recovery images (TR/TE/TI, 8800/130/2200) from 2 different patients with multiple sclerosis (A, B) show the typical Dot-Dash sign. The arrows indicate the dots, whereas the dashes are the hypointense areas between the dots.
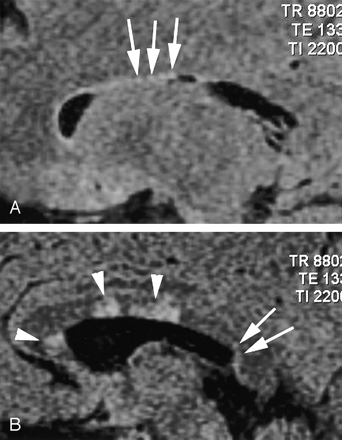
Sagittal fluid-attenuated inversion recovery images (TR/TE/TI, 8800/133/2200) are from a patient with recurrent relapsing multiple sclerosis. A shows the typical Dot-Dash sign (arrows). The patient developed worsening neurologic symptoms, and a follow-up image obtained 7 weeks later (B) showed interval development of confluent callosal and white matter lesions (arrowheads) and an additional Dot-Dash sign in the posterior ependyma.
Image Review
The images were filmed from a workstation after removing all white matter except the corpus callosum and underlying ependyma. All patient and control images were randomly interleaved and numbered by one of the junior authors and then reviewed in consensus by one of the junior authors and an experienced neuroradiologist (the senior author) for the presence or absence of the Dot-Dash sign. All alphanumeric information (except the study number) was removed from the images, and no clinical or demographic information was provided.
Statistical Analysis
The statistical significance was calculated using the chi-square test to compare the 2 groups. A P value of <.01 was considered to be statistically significant. All calculations were performed by using the SPSS software, version 10.0 (Statistical Package for the Social Sciences, Chicago, IL).
Results
The correlation between the Dot-Dash sign and clinical multiple sclerosis was found to be highly significant (P < .001). The Dot-Dash sign was visualized on the images of 32 of 35 patients with clinical multiple sclerosis and on those of 12 of 35 controls. Sensitivity, specificity, and positive and negative predictive values are given in the (Table). Further differentiation into 2 age groups (group I, ≤50 years, n = 55; group II, >50 years, n = 15) revealed a higher sensitivity and specificity for patients <50 years (group I, Table).
Comparison of controls and patients (divided by age)
The 12 control patients whose images showed the Dot-Dash sign had the following clinical characteristics: history of migraine/headache, 5; suspected or known brain/spinal cord tumor, 3; neurofibromatosis, 1; seizure, 1; ataxia, 2.
Discussion
Multiple sclerosis is the most frequent disabling condition affecting the human brain (16). No single feature or diagnostic test is sufficient to make a definitive diagnosis of multiple sclerosis. Characteristic white matter lesions on MR imaging with a concordant clinical presentation are highly suggestive of multiple sclerosis. The most frequently involved structure is the corpus callosum, which is affected early in the disease in the areas adjacent to the subependymal veins lining the ventricular surface (17). The corpus callosum is estimated to be involved in 55%–93% of patients with multiple sclerosis (11, 18, 19).
The large size, early involvement, and histologic and chemical homogeneity of the corpus callosum make it an appealing structure to use for early detection of multiple sclerosis (19). The characteristic appearance on MR imaging is that of periventricular ovoid white matter lesions (13) and subcallosal striations, both of which correspond to the Dawson finger seen by pathologists. In a series of 50 patients, Palmer et al (14) reported a significant association between subcallosal striations and multiple sclerosis. The histopathologic basis of the Dot-Dash sign lies in the fact that early multiple sclerosis lesions start around the subependymal venous wall (10). In contrast to the other imaging findings mentioned previously, the Dot-Dash sign involves only the ependyma.
The Dot-Dash sign was visualized in 32 of 35 patients with multiple sclerosis, diagnosed according to the Poser criteria (15), resulting in a sensitivity of 91.4%; however, images of 12 (34.2%) of 35 control patients also showed the Dot-Dash sign. Because of the frequent nature of ischemic small-vessel white matter changes in the >50-year-old group, we decided to subdivide our study further into 2 age groups. This subdivision resulted in an increased sensitivity (95.7%) and specificity (71.9%) in the younger patients (mean age, 36.7 years; range, 16–50 years) indicating that the Dot-Dash sign is particularly useful in younger patients.
Our study results and methodology were confined to the Dot-Dash sign itself without knowledge of any other brain lesions or clinical history, including the age of the patient. The most sensitive single finding for multiple sclerosis in the MR imaging literature is subcallosal striations (14) with a sensitivity of 94.4% in a study group of 50 patients, which is similar to our results, though our patients may have presented at an earlier stage of the disease. This finding is supported by a difference in the age groups; Palmer et al (14) reported a mean age of 42 years (sensitivity, 94.4%), whereas the mean age of our patients in group I was 36.7 years (sensitivity, 95.7%).
Although the Dot-Dash sign had a highly significant (P < .001) correlation with clinical multiple sclerosis, there was a false-positive rate resulting in a specificity of 65.7% (71.9% in age group I), which is comparable to that of previous signs (9). Fifty percent (6/12) of false-positive Dot-Dash findings were seen in patients with a history of migraine headache (4/12) or in older patients with chronic white matter changes (2/12) (20). The changes of chronic white matter are typically parallel to the ependyma rather than perpendicular (Fig 4), which should allow the differentiation of the 2 disease processes using standard MR imaging criteria. However, in early small-vessel ischemia, focal gliosis may result in a Dot-Dash sign. We also suspect that the same reasoning holds true for the incidence of patients with false-positive findings in the group with migraine headache from mild vasculopathy changes. Chronic white matter changes should be easily differentiated because the 2 disease processes present in different age groups. Migraine headache is the most significant limiting disease process for the Dot-Dash sign. Regardless, the evident power of this study lies in the fact that if the Dot-Dash sign is not seen (especially in the <50-year-old group), then multiple sclerosis is almost certainly excluded (sensitivity in group I, 95.7%).
Sagittal fluid-attenuated inversion recovery image (TR/TE/TI, 8800/130/2200) shows confluent subependymal and callosal white matter hyperintensity (arrows), which is typical of chronic white matter ischemic changes. There is no evidence of either the Dot-Dash sign or subcallosal striations.
One potential limitation of this study is the differentiation of acute disseminated encephalomyelitis from multiple sclerosis. Because the Poser criteria for “definite” multiple sclerosis require repeated incidents in both time and place and because acute disseminated encephalomyelitis is monophasic, some of the control patients with the Dot-Dash sign in fact may have had acute disseminated encephalomyelitis. Because it is likely that acute disseminated encephalomyelitis and multiple sclerosis represent different manifestations of a similar disease process, the Dot-Dash sign may, in fact, represent immune-mediated demyelination rather than multiple sclerosis per se.
In conclusion, the Dot-Dash sign of ependymal irregularity seen on 2-mm sagittal fast fluid-attenuated inversion recovery images increases the sensitivity for diagnosing multiple sclerosis while providing an additional finding of early multiple sclerosis compared with previously described MR imaging criteria. We suggest that the Dot-Dash sign correlates to the known histopathologic perivenular inflammatory process of the ependyma in early multiple sclerosis.
Footnotes
The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Defense or other Departments of the United States Government.
References
- Received December 19, 2004.
- Accepted after revision March 22, 2005.
- Copyright © American Society of Neuroradiology