Abstract
Summary: A 28-year-old man presented with an acute spontaneous dissection of the left posterior communicating artery with associated ipsilateral thalamic and internal capsular infarctions. Positive risk factors included smoking and family history of ischemic heart disease. He was also found to have hyperhomocysteinemia, which has been implicated as a risk factor for spontaneous cervical artery dissection, but to date, no association has been shown with spontaneous intracranial arterial dissection.
Cervical artery dissection accounts for up to 20% of ischemic strokes in young adult patients (1, 2). Spontaneous nontraumatic intracranial arterial dissections are less frequently encountered. Most of the latter occur in the vertebrobasilar circulation, including the posterior cerebral arteries (3). To date, we have not found any report of an isolated dissection or dissecting aneurysm of the posterior communicating artery. In addition to the unusual site of occurrence of the dissection in this case is the associated finding of hyperhomocysteinemia.
Case Report
A 28-year-old man presented with acute loss of consciousness from which he recovered spontaneously but with a degree of memory loss thereafter. There was no history of trauma. Risk factors for vascular disease included smoking as well as a history of familial ischemic heart disease. There was no further relevant history. On examination, his blood pressure was 129/78 mm Hg. Neurologically, he had a pronounced left-sided facial palsy and mild right arm drift. Findings of the remainder of the neurologic examination were normal. A presumptive clinical diagnosis of juvenile stroke was made at this stage.
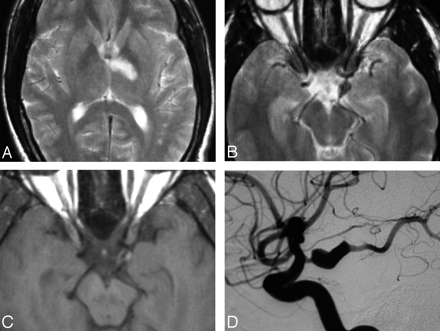
An MR image demonstrated the presence of an acute nonhemorrhagic infarction involving the anterior aspect of the left thalamus and part of the posterior limb of the adjacent left internal capsule (Fig 1A). No subarachnoid blood was noted. Axial scans indicated an oval aneurysmal dilation in the region of the left posterior communicating artery, which showed peripheral bright signal intensity on T1-weighted images, suggesting intramural thrombus (Fig 1B, -C). All findings of cardiac special investigations proved normal. Cerebral digital subtraction arteriography confirmed an isolated fusiform dilation of the left posterior communicating artery (Fig 1D). The posterior communicating artery was dominant, with an associated congenitally hypoplastic P1 segment of the left posterior cerebral artery. The posterior communicating artery was also slightly stenotic just proximal to the dilated segment. No distal emboli were seen. No other abnormalities of the intracranial or extracranial vasculature were found. Further serological investigations showed only a significantly elevated homocysteine level of 68.4 μmol/L (upper limit of normal range, 15 μmol/L). Neither elevation of the cholesterol level nor any other abnormal serologic or hematologic results were found.
28-year-old man with acute loss of consciousness.
A, Axial T2-weighted MR image shows the area of infarction in the anterior aspect of the left thalamus and adjacent internal capsule.
B, Axial T2-weighted MR image shows fusiform dilation of the posterior communicating artery.
C, Axial T1-weighted MR image obtained at the same level as in B shows hyperattenuated signal intensity in the walls of the aneurysm probably due to intramural thrombus.
D, Selective left internal carotid artery digital subtraction arteriogram confirms the presence of a dissecting aneurysm of the posterior communicating artery.
We decided to adopt a conservative approach to his treatment, offering neither direct treatment (endovascular or surgical) of the dissected vessel nor anticoagulation, planning to use the latter only if further thromboembolic complications should occur (these did not happen). For the hyperhomocysteinemia, the patient was placed on oral vitamin B6 and folic acid. He has since recovered from all initial neurologic deficits with no further complications to date.
Discussion
The posterior communicating artery is the proximal remnant of the posterior, dorsal, or caudal division of the embryonic internal carotid artery. It may then either regress as the posterior cerebral artery becomes annexed by the basilar artery or may persist to preferentially give rise to the posterior cerebral artery with corresponding agenesis of the ipsilateral P1 segment. The posterior communicating artery itself gives rise to a set of medial perforating arteries supplying the diencephalic and some mesencephalic territories. These perforators are known variably as the inferior diencephalic, inferior anterior, or anterior thalamic perforators (4). The territories supplied by these vessels include parts of the hypothalamus, the posterior limb of the internal capsule, the anterior and medial ventral aspect of the thalamus, the cerebral peduncle, and the subthalamic optic tract. The extent of supply of these regions by these perforating arteries depends on the degree of balance with other arteries in the same territory, including the anterior choroidal artery and other thalamoperforating arteries. The size of the territory supplied by the posterior communicating artery perforators bears no relation to the diameter of the posterior communicating artery itself (4).
The area of infarction seen in Figure 1A includes the anterior pole of the thalamus and part of the posterior limb of the adjacent internal capsule. Involvement of the latter structure would account for the motor deficits experienced by our patient, whereas his transient memory loss would have been related to involvement of the anterior aspect of the thalamus. This area is an important relay point in the limbic system between the hippocampus via the fornix and the cortex, a pathway critical to memory function. It seems probable that in our patient, the origins of the posterior communicating artery perforators were compromised by the dissecting process that occurred within the wall of the posterior communicating artery itself, producing the infarction in this specific territory.
Homocysteine is a thiol (sulfur)-containing intermediate amino acid formed in vivo from the essential dietary amino acid methionine. Its metabolism is dependent on the function of 3 enzymes: methionine synthetase, cystathionine β-synthetase, and methylenetetrahydrofolate reductase (MTHFR). Vitamins B6, B12, and folic acid are essential co-factors for the function of these enzymes. Homocystinuria is a rare clinical syndrome consisting of neurologic impairment, skeletal abnormalities, ectopia lentis, and thrombotic complications coupled with very high homocysteine levels (>100 μmol/L). It is caused by a genetic defect resulting in a lack of 1 of the 3 principal enzymes involved in homocysteine metabolism and is inherited as an autosomal recessive trait, with only the homozygotes developing the complete clinical syndrome. Approximately half of all homozygotes will develop premature cardiovascular disease by 30 years of age, related to very high serum levels of homocysteine (hyperhomocysteinemia) (5, 6).
Hyperhomocysteinemia is a separate entity with elevated serum levels of homocysteine, generally with concentrations higher than 14 μmol/L but with no excretion of homocysteine in the urine. Mild-to-moderate elevations in the serum homocysteine can occur as a result of a mutation in the gene encoding the enzyme MTHFR, resulting in an elevation of serum homocysteine levels by approximately 20% in homozygotes (6). Dietary deficiencies of folate and vitamins B6, B12, and B2 can also lead to elevated serum homocysteine levels. It is estimated that between 5% and 7% of the general population have hyperhomocysteinemia, which has been identified as a risk factor for various vascular diseases including ischemic heart disease, peripheral vascular disease, venous thrombosis, and stroke (5–9). More recently, hyperhomocysteinemia has been suggested as an independent risk factor for the development of cervical artery dissection, both spontaneous and associated with cervical manipulation (1, 2, 10). To date, we have not yet found any report of an isolated spontaneous intracranial arterial dissection in a patient with hyperhomocysteinemia.
Homocysteine is considered to be toxic to vascular endothelium. Patients with homocystinuria develop extensive arterial intimal thickening and fibrous plaques rich in smooth muscle cells and collagen rather than the more typical fatty deposits seen in atherosclerosis. These, coupled with an increased thrombotic tendency resulting from further endothelial cell changes, result in the early infarctions and death associated with this condition (7). Although it is not known exactly how homocysteine promotes vascular injury, it is thought to be mediated by a combination of inflammatory changes, oxidative stress, and endoplasmic reticulum stress, which duly lead to endothelial cell damage and dysfunction (7). The same effects have been noted in cerebral vessels leading to both small vessel and large vessel intracranial diseases (11–14).
Studies performed in genetically altered mice deficient in certain key enzymes required for homocysteine metabolism resulting in hyperhomocysteinemia have shown endothelial dysfunction, altered vessel mechanics, and vessel hypertrophy at least in the cerebral microcirculation (11, 13, 14). Although recently suggested as a possible risk factor for spontaneous cervical artery dissection, the role of elevated serum homocysteine concentrations in the development of these dissections is unknown and could be part of a complex multifactorial pathomechanism, the first step of which may be homocysteine-induced endothelial damage (1, 2, 15).
Hyperhomocysteinemia has been shown to produce a decrease in the elastin content of arterial walls, mediated by the activation of metalloproteases and serine elastases, resulting in the fragmentation of elastic fibers and degradation of the adjacent extracellular matrix (2). Thus homocysteine may have a direct influence on the elastic layers of the arterial wall, predisposing to or resulting in spontaneous cervical artery dissection. Other factors such as inherited ultrastructural connective tissue or extracellular matrix aberrations may also play an important role in predisposing to spontaneous cervical artery dissection in patients with hyperhomocysteinemia (15). It is conceivable that the same combination of risk factors and pathophysiological effects could result in a number of spontaneous nontraumatic intracranial arterial dissections as well. It would thus be useful to try to correlate the presence of hyperhomocysteinemia and the abnormal C677T MTHFR genotype in patients with nontraumatic intracranial dissections to see whether these could be similarly classified as potential risk factors for intracranial dissections as well.
References
- Received October 25, 2004.
- Accepted after revision October 29, 2004.
- Copyright © American Society of Neuroradiology