Abstract
BACKGROUND AND PURPOSE: MR imaging may help in predicting hemorrhagic transformation (HT) in acute ischemic stroke. Our purpose was to determine whether the lesion volumes on diffusion-weighted (DW) imaging, apparent diffusion coefficient (ADC) values, and early parenchymal enhancement are predictive of HT and to investigate the mechanism of the enhancement.
METHODS: We retrospectively examined 55 patients with acute ischemic stroke who underwent gadolinium-enhanced MR imaging within 6 hours of symptom onset and follow-up CT or MR imaging within 72 hours. Intravenous thrombolysis was performed in 15 patients. DW imaging lesion volumes and ADC values were compared between patients with and those without HT. ADCs and perfusion parameters were compared between lesions with and those without parenchymal enhancement.
RESULTS: Nineteen (34.5%) patients had HT (14 with hemorrhagic infarction, five with parenchymal hematoma). Patients with HT had decreased mean ADCs and large lesion volumes on DW imaging, but differences were not significant (P > .05). HT occurred in five patients (100%) with parenchymal enhancement, which corresponded to the site of HT. In enhancing lesions, the ADC ratio (0.76 ± 0.06) was slightly higher and the delay in time to peak (0.10 ± 2.79) was less than respective values in the rest of the ischemic lesion (0.66 ± 0.06 and 8.79 ± 4.86, respectively; P = .068).
CONCLUSION: Early parenchymal enhancement is highly specific for HT and may be associated with early reperfusion and damage to the blood-brain barrier in ischemic tissue. DW imaging lesion volumes and ADC values had no strong relationship with HT.
Intravenous recombinant tissue-type plasminogen activator (r-tPA) or intra-arterial thrombolytic therapy substantially improves patients’ neurologic outcomes but increases intracerebral hemorrhage (1–5). Therefore, the identification of risk factors leading to hemorrhage is crucial for effective and safe thrombolytic therapy. Various clinical risk factors for hemorrhagic transformation (HT) in acute ischemic stroke have been documented (4–11). In the European Cooperative Acute Stroke Study I trial, the large extent of hypoattenuation on CT was established as a predictive factor for symptomatic hemorrhage and for the poor outcome after intravenous r-tPA thrombolytic therapy (12). However, this finding remains controversial in patients treated within 3 hours (11, 13). Low apparent diffusion coefficient (ADC) values, old microbleeds, and early parenchymal enhancement have been suggested as potential MR imaging predictors of hemorrhage (14–23). Reports suggest that stroke MR imaging may be useful in identifying risk factors of hemorrhage that may not be identified on CT. Results of several animal studies suggest that early parenchymal enhancement is observed after reperfusion and that it may enable early prediction of HT (20–22). In their study, Vo et al (23) suggested that the early parenchymal enhancement may be a good predictor of symptomatic HT in patients with acute ischemic stroke. However, to our knowledge, the value of various MR imaging features for the prediction of hemorrhage has not been established in acute ischemic stroke.
The purpose of this study was to determine whether lesion volume on diffusion-weighted imaging (DW), ADC values, and early parenchymal enhancement are predictive of HT in acute ischemic stroke and to investigate the mechanism of early parenchymal enhancement.
Methods
Patient Selection
Reviewing the medical records, we retrospectively selected 415 patients who presented within 6 hours after the onset of acute ischemia between February 1997 and August 2003. We enrolled 55 who met the following criteria: (1) confirmed acute infarction of the middle cerebral artery (MCA) territory; (2) MR imaging, including gadolinium-enhanced T1-weighted and DW imaging, within 6 hours of symptom onset; and (3) follow-up nonenhanced CT or MR imaging, with conventional gradient-echo (GRE) or echo-planar gradient-echo imaging (EPI-GRE), within 72 hours. If indicated, intravenous rt-PA 0.9 mg/kg (alteplase, maximum of 90 mg) was administered by infusing 10% as a bolus followed by a constant infusion of the remaining 90% for 60 minutes between the initial nonenhanced CT and MR imaging studies.
Imaging Methods
CT scans were obtained before MR imaging in all patients. MR images were obtained with a 1.5-T unit (Signa; GE Medical Systems, Milwaukee, WI). The typical stroke-imaging protocol included DW imaging, perfusion-weighted imaging (PWI), T2*-weighted GRE imaging, and Gd-enhanced T1-weighted imaging, and time-of-flight MR angiography of the intracranial arteries. DW imaging was performed with 20 sections and b values of 0 and 900 or 1000 s/mm2. Averaged DW images were generated online by averaging three orthogonal-axis images. Imaging parameters for DW imaging were as follows: TR/TE = 6500/96.8, matrix = 128 × 128, FOV = 24 × 24 or 28 × 28 cm, section thickness = 5 mm, and intersection gap = 2 mm. In all patients, enhanced axial T1-weighted images were obtained after PWI; the parameters were as follows: matrix = 256 × 192, FOV = 24 × 24 cm, section thickness = 5 mm, intersection gap = 2 mm, and one excitation. PWI was performed with EPI-GRE sequences with these parameters: TR/TE = 2000–2500/50–60, flip angle = 90°, FOV = 24 × 24 cm, matrix =128 × 128, section thickness = 5 mm, and intersection gap = 2 mm. This sequence was performed the injection of gadopentetate dimeglumine 0.2 mmol/kg (Magnevist; Schering, Berlin, Germany) at a rate of 4 mL/s with an MR imaging-compatible power injector (Spectris; Medrad, Pittsburgh, PA). The bolus of contrast material was followed by a 15-mL bolus of saline at the same injection rate. A series of images (8–10 sections, 40–50 images per section) was obtained before, during, and after administration of the contrast agent. Perfusion maps of relative cerebral blood volume (rCBV), time to peak (TTP), and relative cerebral blood flow (rCBF) were generated offline at a workstation. Details of the postprocessing method for perfusion maps were described previously (24).
Data Processing and Analysis
One neuroradiologist manually drew the region of interest (ROI) for the hyperintense lesion on each DW imaging section. These ROIs were transferred to the ADC maps. Lesion volumes on DW imaging and ADC values of voxels in the ROIs were calculated. ADC values in each DW imaging lesion were collected in all patients and divided patients into four groups: ≤250 × 10−6, ≤350 × 10−6, ≤450 × 10−6, and ≤550 × 10−6 mm2/s. The percentage and absolute number of voxels within the group were obtained. The threshold for ADC values was 1.2 × 10−3 mm2/s to minimize the partial-volume effect on CSF.
Two neuroradiologists who were blinded to the follow-up images and clinical information independently reviewed the MR images to determine the presence of early parenchymal enhancement. When a discrepancy occurred, the decision was made by a consensus. Early parenchymal enhancement was defined as a hyperintense area on the initial Gd-enhanced T1-weighted image, which was noted as the area of the hyperintense lesion on DW images. In patients with early parenchymal enhancement, mean ADCs and perfusion parameters, including TTP, rCBV, and rCBF, was obtained in the enhancing area and in the rest of the ischemic lesion. For quantitative ROI measurement in patients with early parenchymal enhancement, enhanced T1-weighted images and PWIs were spatially coregistered to the DW imaging (EPI T2-weighted imaging, b = 0 s/mm2) to superimpose the ROIs delineated on enhanced-T1 images by using SPM2 software (Wellcome Department of Cognitive Neuroscience, www.fil.ion.ucl.ac.uk/spm/).
HT was defined and classified into four subtypes, as previously described (3, 14, 25): hemorrhagic infarct type 1, which was small petechiae along the margins of the infarct; hemorrhagic infarct type 2, which was more confluent petechiae within the infarcted area but without space-occupying effect; parenchymal hematoma type 1, which was hematoma in less than 30% of the infarcted area with some space-occupying effect; and parenchymal hematoma type 2, which was hematoma in more than 30% of the infarcted area with substantial space-occupying effect or any hemorrhagic lesion outside the infarcted area. Symptomatic hemorrhage was defined as clinical deterioration with a National Institutes of Health Stroke Scale (NIHSS) score of more than 3 likely due to hemorrhage. Two neuroradiologists identified HT by consensus on follow-up MR images (n = 47; with GRE images in 24 patients and EPI-GRE images in 23) or CT scans (n = 8) within 72 hours of symptom onset.
Review of Clinical Data
We reviewed the patients’ clinical data: baseline neurologic deficits, as assessed by using the NIHSS score; history of hypertension; use of an antiplatelet agent (aspirin) or an anticoagulant (warfarin); cardioembolic stroke risk factors, such as atrial fibrillation; and history of diabetes.
Statistical Analysis
Statistical analysis was performed by using commercially available software (SPSS-PC, version 10.0, 1999; SPSS, Chicago, IL). Patients were divided into two groups according to the presence or absence of HT. Differences in MR imaging variables between the groups were assessed by using the Student t test for continuous variables. Nominal clinical variables between the groups were compared by using χ2 test. Diffusion and perfusion parameters in the ischemic lesion were compared between lesions with and those without early parenchymal enhancement by using the Wilcoxon signed ranks test. A P value less than .05 was considered to indicate a statistically significant difference.
Results
Fifty-five patients (29 men, 26 women; mean age, 68.8 ± 10.8 years; age range, 50–91 years) were selected. The mean interval from the onset of their symptoms to MR imaging was 4.4 ± 1.0 hours, with a range of 2.2–6.0 hours. The mean time to follow-up imaging was 1.27 ± 0.2 days, ranging from 1 to 3 days. Intravenous r-tPA therapy was performed in 15 patients after MR imaging. HT was identified in 19 patients (34.5%) at follow-up: hemorrhagic infarct in 14 patients and parenchymal hematoma in five. Symptomatic hemorrhage was observed in one patient.
Table 1 summarizes the results of MR imaging. Compared with patients without HT, patients with HT had a lower mean ADC, a larger lesion volume on DW imaging, and more voxels with low ADC. However, the difference was not significant (P > .05). Similarly, among the 15 patients treated with r-tPA, those with HT had larger DW imaging lesion volumes and lower ADC values than those of patients without HT. However, the differences for the DW imaging variables were not significant (P > .05).
DWI lesion volumes, ADC values, and early parenchymal enhancement
Early parenchymal enhancement was observed in five patients (9%). All patients with this enhancement had HT on follow-up imaging. Two received intravenous r-tPA therapy and had a parenchymal hematoma type 1 or a hemorrhagic infarct type 1 in the left basal ganglia. The others had a parenchymal hematoma type 1, hemorrhagic infarct type 2, or hemorrhagic infarct type 1 in the left parietal lobe, left frontal lobe, and left basal ganglia, respectively. No early parenchymal enhancement was identified in the patients without HT (P = .003). The HT sites corresponded to those with parenchymal enhancement in all patients. No symptomatic HT developed in patients with early parenchymal enhancement.
Diffusion and perfusion parameters of the enhancing lesion were quantitatively analyzed in four patients with early parenchymal enhancement in whom PWI was available (Table 2). Mean ADC values and ADC ratios of enhancing lesions were slightly higher than values in the rest of the ischemic lesion, but the difference was not significant (P = .068 and P = .066, respectively). The mean TTP delay was lower in lesions with early parenchymal enhancement than in lesions without enhancement (P = .068). Although the perfusion parameters did not significantly differ between the groups, the lesion with early parenchymal enhancement had a TTP delay of less than 2 seconds, an increased rCBV (ratio >1.19), and an increased rCBF (ratio >1.16) in three of four patients.
ADC and perfusion parameters of four enhancing lesions
The mean initial NIHSS score of 55 patients was 15.0 ± 5.6. In patients with HT, the score was 13.2 ± 6.1, and in patients without HT, it was 9.8 ± 5.7 (P = .043). Subsequent HT was noted in the following patients: 12 of 30 with hypertension (P > .05), in six of 14 receiving an anticoagulant or an antiplatelet agent (in three of six taking warfarin and in three of eight taking aspirin, P > .05), in 12 of 24 with atrial fibrillation (P = .047), and in 10 of 22 with a history of diabetes (P > .05).
Discussion
ADC values and DW imaging lesion volumes were not strongly associated with HT, and early parenchymal enhancement was significantly associated with HT. Early parenchymal enhancement was 29% sensitive but 100% specific for the development of HT.
Clinical MR studies showed that the ADC value of ischemic tissue was predictive of HT (14–17). Although a lesion with low ADCs may have severe ischemic damage that predisposes the patient to HT, especially after thrombolysis, our data from a small number of patients suggest that the ADC values themselves are not as reliable as early parenchymal enhancement for predicting HT. This may be because of a fogging effect on the ADC value, as reflected by the slightly higher mean ADC ratios found in our patients with parenchymal enhancement. This fogging effect may be the result of early vasogenic edema with increasing T2 signal intensity 2–6 hours after stroke, when the ADC decline has stabilized, or the result of early reperfusion (26–29). In three of our patients, the region with parenchymal enhancement and less severely depressed ADC had a shortened TTP, an increased CBV, and an increased CBF, all of which were consistent with hyperperfusion (Fig 2).
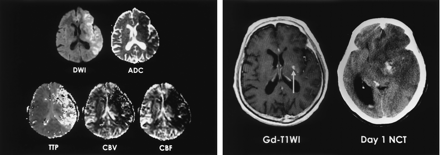
A 74-year-old man with right hemiparesis and aphasia. Intravenous r-tPA was administered after nonenhanced CT was performed 2.5 hours after symptom onset.
A, DW image and ADC map obtained 3 hours after CT show ischemic edema in the left MCA territory. TTP, rCBV, and rCBF maps show perfusion abnormality.
B, Gd-enhanced T1-weighted image (T1WI) shows a focal enhancing area in the left basal ganglia (arrow). Follow-up nonenhanced CT scan (NCT) depicts a small parenchymal hematoma in the left basal ganglia that corresponds to the enhancing area.
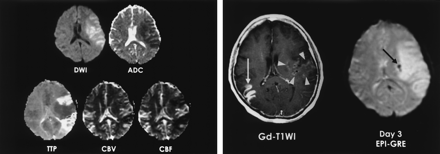
A 57-year-old woman with right hemiparesis and aphasia. Intravenous r-tPA was administered after nonenhanced CT was performed 1.5 hours after symptom onset.
A, DW image and ADC map obtained 3.7 hours after CT show ischemic edema and perfusion abnormality in the left MCA territory. TTP and rCBF maps show reperfusion in the area of parenchymal enhancement.
B, Gd-enhanced T1-weighted image (Gd-T1WI) shows parenchymal enhancement in the left perisylvian cortex and basal ganglia (arrowheads). Enhancing lesion in the right temporal lobe (white arrow) indicates subacute infarction. Patient had atrial fibrillation and infarction 2 months earlier. Follow-up EPI-GRE image shows focal hemorrhage (hemorrhagic infarct type 1) in only the left basal ganglia (black arrow). No hemorrhage is present in the rest of area of parenchymal enhancement in the left MCA territory and in right temporal lobe on the enhanced T1-weighted image.
Our finding of a correlation between parenchymal enhancement and HT was consistent with those of previous animal and human studies (21–23). Contrast enhancement before treatment is predictive of the severity of hemorrhage and has a good correlation with the subsequent severity of HT, as shown in an animal study (22). In one study, two of three patients with early parenchymal enhancement after r-tPA treatment had symptomatic hemorrhage at follow-up (23). Because r-tPA can aggravate the rapid breakdown of the microvascular barrier (30), early parenchymal enhancement might be a potential risk factor for posttreatment symptomatic hemorrhage. In contrast to patients in the study by Vo et al (23), our patients with early parenchymal enhancement did not develop symptomatic hemorrhage after r-tPA therapy; therefore, the relevance of the findings to symptomatic hemorrhage remains uncertain (25, 31, 32).
Various clinical data, such as baseline NIHSS score, history of hypertension, anticoagulant use, and cardioembolic stroke risk factors (e.g., atrial fibrillation, history of diabetes) have been suggested as risk factors for HT in patients with acute ischemic (4, 6–11). In our study, baseline NIHSS scores and atrial fibrillation were significantly associated with subsequent HT, comparable to results of prior studies. However, other variables were not associated, possibly because of relatively small number of patients.
Although the different imaging parameters for PWI were used, the difference of scan parameters will not significantly affect the measured values of TTP delay, relative CBV, or relative CBF because we measured only relative values (difference of TTP value or ratios), and the measured values were obtained from the fitted curve by the same postprocessing software.
This study had a couple limitations. First, we were unable to analyze the relationship between MR imaging features and symptomatic hemorrhage because of the lack of subjects with symptomatic hemorrhage. Second, this study was retrospective and did not include treated patients who underwent MR imaging before thrombolytic therapy. Therefore, further prospective study should be conducted with a large number of patients to validate early parenchymal enhancement as a valuable predictor of HT and to determine the clinical importance of early parenchymal enhancement in terms of thrombolytic therapy in patients with acute ischemic stroke.
Conclusion
Early parenchymal enhancement was uncommon (9%) in patients with acute MCA infarction within 6 hours of symptom onset, but it was highly specific for HT. This enhancement may be associated with early reperfusion, as well as damage to the blood-brain barrier in the ischemic tissue. DW imaging lesion volume and ADC value were not strongly associated with HT.
Footnotes
Supported by a grant from Guerbet Korea, Seoul, Korea.
References
- Received July 7, 2004.
- Accepted after revision October 5, 2004.
- Copyright © American Society of Neuroradiology