Abstract
BACKGROUND AND PURPOSE: Besides the severity of carotid artery stenosis, atherosclerotic plaque composition is an important determinant of cerebral symptoms. We analyzed the relationship between the composition of the atherosclerotic plaque at the carotid artery bifurcation and ipsilateral ischemic cerebral lesions on MR imaging.
METHODS: Forty-one patients with symptomatic carotid artery stenosis (>70%) underwent black-blood, fast spin-echo imaging of the carotid artery and turbo fluid-attenuated inversion recovery (t-FLAIR) imaging of the brain. Plaque regions with a relative decrease in signal intensity in the plaque from proton density-weighted (TE = 14 ms) to T2-weighted (TE = 50 ms) imaging were considered to be lipid cores. We assessed the number and location of infarcts in the ipsilateral cortex, basal ganglia, and centrum semiovale, and hyperintense white matter lesions on t-FLAIR images.
RESULTS: Lipid in the atherosclerotic plaque at the carotid bifurcation was seen in 25 patients. Ipsilateral infarctions were seen in 22 (54%); most often, it involved the centrum semiovale. Patients with a lipid core had an ipsilateral infarct more often than patients without a lipid core (68% vs. 31%; P = .03). Centrum semiovale infarcts were more frequent (56% vs. 25%, P = .06) and the median number of centrum semiovale infarcts was higher P = .04) in patients with a lipid core than in patients without a lipid core.
CONCLUSION: Ischemic cerebral lesions were common in patients with symptomatic carotid artery disease. Plaque composition, as assessed with MR imaging, is related to the presence and extent of ischemic cerebral lesions.
The severity of carotid stenosis is strongly related to ipsilateral cerebral infarction, and it is used as parameter in deciding which patients may benefit from treatment of the carotid lesion (1, 2). However, a large proportion of cerebral infarcts may be caused by thromboembolism. This observation suggests that the degree of carotid stenosis is only indirectly related to the occurrence of cerebral infarction and that it does not capture the essence of the process. Besides the severity of stenosis, plaque composition is considered an important determinant of symptoms. The concept of unstable or vulnerable plaque that may rupture and release thromboembolic material has been postulated for the coronary arteries (3–5), and it may also be applicable to the carotid arteries. This vulnerable plaque contains a large necrotic lipid core covered by a thin or disrupted fibrous cap (6–8). Information concerning the composition of the plaque may enhance predictions of the infarct risk that are based on the degree of carotid stenosis.
Imaging of carotid plaque components in vivo has been performed with ultrasonography (US) (9, 10). However, histologic evaluation demonstrates no clear relationship with different plaque components, and interobserver variability is high (11, 12). Therefore, a more quantitative method with less interobserver variability, the gray-scale median (GSM), was introduced to characterize the plaque (12, 13). GSM is positively related to both clinical symptoms of cerebral ischemia and ischemic lesions demonstrated on CT (13–15).
High-resolution MR imaging has emerged as a potential technique for atherosclerotic plaque imaging (16, 17). In addition, MR imaging has proved its superiority over CT in the analysis of ischemic cerebral lesions (18). To test the concept of a vulnerable plaque leading to an increased risk for cerebrovascular events, we analyzed the relationship between the composition of the carotid atherosclerotic plaque and ipsilateral ischemic cerebral lesions in symptomatic patients on MR imaging.
Methods
Patients
Between November 2000 and July 2003, 64 patients (13 women, 51 men; median age, 68 years; age range, 44–86 years) with symptomatic carotid artery stenosis (>70%) scheduled for endarterectomy or carotid stent placement underwent MR imaging of both the carotid artery and the brain.
This study was approved by the local institutional review board, and patients gave written informed consent. The patients were screened for cardiovascular risk factors, including hypertension, hypercholesterolemia, diabetes mellitus, and smoking. The severity of carotid artery stenosis as defined by using the North American Symptomatic Carotid Endarterectomy Trial Collaborators criteria (1) was based on selective digital subtraction angiography. A division was made between 70–89% and 90–99% stenosis.
Imaging Protocol
MR imaging was performed on a 1.5-T system with a gradient amplitude of 40 mTm−1 and a maximum slew rate of 150Tm−1s−1 (Signa CV/i; GE Medical Systems, Milwaukee, WI). To obtain high-resolution images of the symptomatic carotid artery, a dedicated phased-array coil (19) was used. The brain was imaged with a quadrature head coil (GE Medical Systems).
Patients were imaged within 3 days before carotid intervention. Table 1 shows the imaging protocol for the carotid artery. First, a two-dimensional time-of-flight sequence of the carotid bifurcation was performed. The caudal coordinates of the bifurcation on the images were used to plan eight consecutive axial 3-mm proton density-weighted (TE = 14 ms) and T2-weighted (TE = 50 ms) black-blood, fast spin-echo sections with the carotid artery in the center of the image (Table 1). The first section was planned for just below the bifurcation; the next sections were cranial, without intersection spacing. To reduce the imaging time, T2-weighted images were acquired only at the levels at which atherosclerotic disease was seen on the proton density-weighted images (19). MR imaging of the brain was subsequently performed. The MR imaging protocol consisted of a t-FLAIR sequence (Table 1).
Imaging protocol
Definitions and Analyses
Previous studies with 1.5–3T units demonstrated that lipid core has a shorter T2 than that of fibrous tissue, both in vitro (20–23) and in vivo (24, 25). Therefore, lipid has a relative signal intensity drop on proton density-weighted images versus T2-weighted images in comparison to fibrous tissue. Shinnar et al (26) confirmed these findings in an ex vivo study with a 9.4-T a machine and reported that lipid had high intensity on proton density-weighted images and lower intensity on T2-weighted images. Therefore, a distinct region in the atherosclerotic plaque, which showed a relative decrease in signal intensity to fibrous tissue from proton density- to T2-weighted images (from hyperintense to intermediate or hypointense or from intermediate to hypointense) was considered to represent lipid core (Figs 1 and 2).
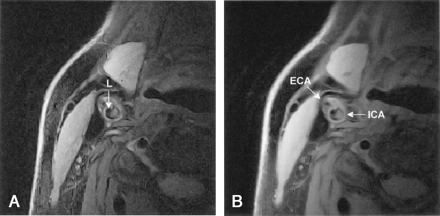
Axial lack-blood fast spin-echo images of the internal carotid artery (ICA) and external carotid artery (ECA). Comparison of images reveals no changes in relative signal intensity of the plaque. Hyperintense region is fibrous tissue, and hypointense spots are calcifications.
A, Proton density-weighted image. L = lumen.
B, Corresponding T2-weighted image. Plaque in the ICA is mainly hyperintense, with hypointense spots.
Axial lack-blood fast spin-echo images of the ICA (arrow). Plaque in the ICA is hyperintense. L = lumen.
A and B, Proton density-weighted (A) and corresponding T2-weighted (B) images. Large region of the plaque has intermediate signal intensity, with hyperintense edges.
C and D, Another patient. Proton density-weighted (C) and corresponding T2-weighted (D) images. Plaque in the ICA has a large region of intermediate signal intensity, which is relatively decreased in D, while the rest of the plaque remains hyperintense.
Two observers (M.O., A.v.d.L.) analyzed the MR images independently, blinded to the results of the brain MR imaging. Differences were solved by consensus and these results were correlated with the cerebral lesions. The two observers assessed the quality of the MR images of the carotid artery and classified them by consensus as good, moderate, or poor. Only studies with good image quality were analyzed further. The signal intensity of plaque components at the most stenotic region was assessed relative to the signal intensity of surrounding muscles on proton density- and T2-weighted images. The signal intensity was classified as hyperintense, intermediate, or hypointense.
FLAIR images of the brain were analyzed for the presence and number of cortical infarcts, subcortical infarcts, and hyperintense white matter lesions in the ipsilateral hemisphere. Cortical infarctions were defined as focal atrophy of the cortex and underlying white matter, characterized by signal intensity similar to CSF. The atrophy was surrounded by a hyperintense region (Fig 3A). Subcortical infarcts were defined as hypointense lesions with the signal intensity of CSF. This hypointense lesion may have been surrounded by a hyperintense ring (Fig 3B). A division was made on the basis of the location of infarcts in basal ganglia and centrum semiovale. Hyperintense white matter lesions were defined as hyperintense regions ≥3 mm (Fig 3C). Two experienced neuroradiologists (ZF, A.v.d.L.) analyzed the images, and differences were solved by consensus. The neuroradiologists were blinded to the results of the carotid plaque analysis. Carotid plaque and brain were analyzed with a time interval of 4 weeks. The presence and number of cerebral lesions in patients with and without lipid core were compared.
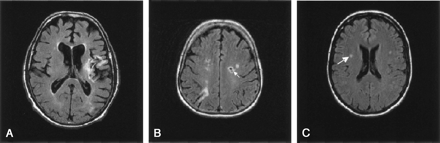
t-FLAIR images of the brain.
A, Cortical infarct in the territory of the left middle cerebral artery.
B, Centrum semiovale infarct (arrow) in the left hemisphere.
C, White matter lesion (arrow) in the right hemisphere.
Statistical Analysis
Statistical analysis of the data was performed with software (SPSS version 11.0.1; SPSS Inc., Chicago, IL). Differences between categorical data were analyzed with the Fisher exact test, and continuous data, with the Mann-Whitney test. Mantel-Haenszel χ2 test was used to assess the influence of potential confounders, such as severity of stenosis, on the relationship between lipid core and cerebral lesions. A P value <.05 was considered to indicate a statistically significant difference. No adjustments for multiple comparisons were made.
Results
MR images of the carotid artery were of good quality in 41 patients (10 women, 31 men; median age, 67 years; age range, 44–86 years). Images of the carotid artery of 22 patients showed motion blur or a low signal-to-noise ratio and were therefore rated as moderate (n = 15) or poor (n = 7) quality; these patients were excluded from analysis. One patient was excluded because of severe cerebral leukoaraiosis of the brain, which hampered analyses of hyperintense cerebral lesions. Clinically, 19 patients presented with an infarct, and 25 presented with a transient ischemic attack.
We found no statistically significant relationships between ipsilateral cerebral lesions (cortical and subcortical infarcts and white matter lesions) and patient characteristics (i.e., age, sex, smoking habits, hypertension, hypercholesterolemia, diabetes, or severity of stenosis).
Plaque Composition
All plaques revealed hyperintense (fibrous) plaque components in all proton density- and T2-weighted images. A lipid core, as indicated by a relative drop in signal intensity in the plaque, was seen in 25 patients.
Ipsilateral Cerebral Lesions
Nine patients had a cortical infarct. Three basal ganglia infarcts in two patients and 45 centrum semiovale infarcts in 18 patients were seen. The number of centrum semiovale infarcts in these 18 patients ranged from one to six. All patients with a basal ganglia infarct had also one or more centrum semiovale infarcts. In 34 patients, 124 hyperintense white matter lesions were revealed (range, one to 12).
Relationship between Plaque Composition and Cerebral Lesions
Table 2 summarizes results from the analysis of lipid core in the atherosclerotic plaque versus cerebral lesions. The number of cortical infarcts was not different in patients with and in those without a lipid core. Centrum semiovale infarcts were more frequent (56% vs. 25%, P = .06) and the number of centrum semiovale infarcts was significantly higher (P = .04) in patients with a lipid core than in the patients without a lipid core. Combining all infarcts, we found that infarcts were more frequent in patients with a lipid core in the atherosclerotic plaque than in patients without a lipid core (P = .03). The presence of a lipid core was significantly associated with any cerebral infarction on MR imaging (crude odds ratio, 4.7; 95% confidence interval: 1.0, 22.9). After an adjustment for severity of carotid stenosis, the association was unaffected (Mantel-Haenszel odds ratio, 4.2; 95% confidence interval: 0.94, 22.7). The presence and number of hyperintense white matter lesions was not different in patients with and in those without a lipid core.
Relationship between plaque composition and cerebral lesions
Discussion
The composition of the atherosclerotic plaque at the carotid artery is considered an important determinant of cerebrovascular events. Histologic studies have revealed that lesions associated with the development of ischemic symptoms contain a large lipid core and a thin fibrous cap (6). Therefore, in vivo assessment of plaque composition is a step forward in the prevention of cerebral ischemia. A cross-sectional study in which clinical symptoms were related to atherosclerotic plaque composition, as assessed with MR imaging, showed that patients with ruptured fibrous caps were 23 times more likely to have a transient ischemic attack or stroke compared with patients with thick fibrous caps (17). To our knowledge, our study is the first to analyze the relationship between MR imaging-assessed plaque composition and MR imaging-assessed brain lesions: Lipid in the atherosclerotic plaque at the carotid artery was related to the presence of cerebral infarcts.
Evaluation of cerebral damage by means of imaging-based analysis differs from clinical analysis. On the one hand, symptomatic patients do not necessarily have structural brain damage; on the other hand, asymptomatic patients may have ischemic lesions on imaging studies. MR imaging is superior to CT in the assessment of these ischemic lesions (27).
The relationship between plaque characteristics and cerebral lesions has been studied with US (plaque) and CT (brain) (15). Geroulakos et al (14) examined patients with >70% stenosis who were mainly symptomatic and found that focal cerebral infarctions visible with CT were more frequent in patients with echolucent plaques, which were assumed to be lipid. This finding was confirmed in subsequent studies in which the GSM was assessed as a measure of echogenicity. Investigators found that a low GSM was related to more ipsilateral focal cerebral infarctions on CT (28, 29). Another study in asymptomatic patients with stenosis <60% revealed that patients with heterogeneous, mainly echolucent, plaques had an increased incidence of new CT- and MR imaging-detected lesions during follow-up (30). The number of lesions on MR imaging was greater than the number of lesions on CT (30). Overall, echolucent plaques (low GSM), which probably contain lipid or thrombus, are related to CT-detected focal cerebral ischemia. Our finding of a relationship between a relative decrease in signal intensity (an indicator of lipid) and cerebral infarcts is in accordance with previous results.
MR imaging can be used to differentiate types of cerebral lesions that are thought to be caused by different pathophysiologic mechanisms. Infarcts involving the cortex are caused by thromboembolic occlusion of the main cerebral arteries or cortical branches. Basal ganglia infarcts or so-called lacunar infarcts are caused by diseased small vessels, which result in occlusion of the deep perforating arteries. The underlying cause of centrum semiovale infarcts is still a matter of debate (31–33), but evidence suggests that large-vessel disease with thromboembolic release of material plays a role in their pathogenesis. The clinical characteristics of patients with centrum semiovale infarcts differ from those of patients with basal ganglia infarcts, with higher frequencies of carotid artery disease in the former (32, 34). For this reason, basal ganglia, centrum semiovale, and cortical infarcts were analyzed separately and then combined. The relationship between centrum semiovale infarcts and a lipid core in the atherosclerotic plaque points in the direction of large-vessel disease instead of small-vessel disease as the underlying cause of these infarcts. Centrum semiovale infarcts are less devastating than cortical infarcts. However, when centrum semiovale infarcts are indicators of end-branch infarctions like cortical infarctions, detection of vulnerable plaque with MR imaging may help in preventing serious neurologic complications. A large population-based study atherosclerotic disease in the carotid artery showed relative risks of 3.2 for nonlacunar infarcts and 10.8 for lacunar infarcts (35). Therefore, one may argue that our findings should not be explained by using a different pathophysiologic mechanism between basal ganglia infarcts and centrum semiovale infarcts, but rather, by using the small number of basal ganglia infarct, which prevented the demonstration of a significant difference in patients with and in those without a lipid core.
Hyperintense white matter lesions were frequently seen, and no relationship was found with plaque composition. These lesions have been related to cerebrovascular risk factors, but direct demonstration for an ischemic origin of these lesions is lacking (36).
This study had several limitations. First, decreased signal intensity on T2-weighted images is considered to represent lipid core. However, hemorrhage and thrombosis may also cause this decrease in signal intensity on T2-weighted images compared with proton density-weighted images because of stronger susceptibility effect with the longer TE. Nevertheless, besides the lipid core, hemorrhage and thrombus are also constituents of the vulnerable plaque (37, 38). Second, we examined only symptomatic patients with severe (>70%) stenosis. Asymptomatic patients, patients with restenosis, and patients with carotid occlusion were not included. Investigating whether plaque composition is related to brain infarcts in patients without symptoms or in those with less stenosis would be important. Third, despite the applicability of high-resolution MR imaging in the assessment of plaque composition, this method has some practical limitations. MR imaging is vulnerable to motion artifacts because of the (generally) long acquisition time. Therefore, in this study, 23 patients with inadequate image quality were excluded from the analysis. Contraindications to MR imaging, particularly claustrophobia, can exclude also a considerable number of patients. Fourth, the relationship between lipid and cerebral lesions may have been confounded by severity of the ipsilateral carotid stenosis. Patients with a lipid core may have had more severe carotid stenosis resulting in hemodynamic limitations and causing internal watershed infarctions (39). However, the observed relationship remained present after an adjustment for the severity of carotid stenosis. Last, this study had a cross-sectional design and provided evidence that plaque composition (lipid core) was related to chronic ischemic cerebral lesions. Prospective follow-up studies should be conducted to determine the predictive value of plaque composition in terms of the risk of new cerebral lesions.
Conclusion
Results of this cross-sectional study demonstrated a correlation between the presence of lipid in the plaque in the carotid artery and the incidence of ipsilateral cerebral infarcts. Follow-up studies are warranted to assess the prognostic value of MR imaging-assessed plaque composition with regard to occurrence of new cerebral infarcts.
References
- Received July 9, 2004.
- Accepted after revision September 15, 2004.
- Copyright © American Society of Neuroradiology