Abstract
BACKGROUND AND PURPOSE: Occlusion of major cerebral arteries is the primary source of tissue damage in ischemic stroke and the target of thrombolytic therapy. We hypothesized that large infarcts in more proximal vascular occlusions correspond with substantially increased ischemic lesions shown on initial apparent diffusion coefficient (ADC) maps.
METHODS: Initial ADC lesions in 120 patients with acute ischemic stroke were analyzed within 6 hours of stroke onset. Patients were categorized on the basis of vascular occlusion, as shown on MR angiography. Lesion volumes were determined by using manual delineation (ADCman) and a threshold method for ADC values (<550 × 10−9 mm2/s−1, ADC<550). Infarct volumes were analyzed by using T2-weighted (n = 109) or CT (n = 11) images obtained on days 5–8.
RESULTS: Median lesion volumes for ADC<550, ADCman, and infarcts, respectively, were as follows: proximal internal carotid artery (ICA)/middle cerebral artery (MCA) occlusions, 10, 23, and 32 cm3; carotid-T occlusions, 11, 37, and 138 cm3; MCA trunk occlusions, 11, 27, and 44 cm3); and MCA branch occlusions 8, 27, and 21 cm3. Initial ADC lesion volumes were different only between the carotid T and the MCA branch (P < .05). On days 5–8, infarct volumes decreased from proximal to distal sites (P < .05), with the exception of MCA trunk versus proximal ICA/MCA occlusions. Recanalization rate in carotid-T occlusion was significantly lower than those of all other occlusion types.
CONCLUSION: Initial ADC lesions can be small, even in patients with proximal vascular occlusions. These patients develop considerably large infarctions, suggesting a high potential for infarct growth. This growth might be averted with improved early recanalization of proximal vascular occlusions.
Occlusion of major cerebral arteries is the primary source of tissue damage in ischemic stroke and the target of thrombolysis therapy. The degree of collateralization and thus the degree and extent of the perfusion deficit determines the volume of the initial metabolic impairment at each site of vascular occlusion. Furthermore, the infarct volume depends on the specific rate and time of vessel recanalization (1–3). Using digital subtraction angiography, several groups have described the relation of the site of vascular occlusion to the rate of recanalization with thrombolysis (4–6). As a complement to these reports, an assignment of the initial ischemic lesion to the site of vascular occlusion—with its specific recanalization rates—would improve our understanding of stroke pathophysiology. In this study, we sought to determine whether poor outcomes with proximal vascular occlusions are already determined at time of diagnosis or whether they are the result of the subsequent poor recanalization rates.
Multiparametric MR imaging allows us to determine the type of vascular occlusion and permits a sensitive evaluation of initial metabolic impairment. Diffusion-weighted imaging (DWI), perfusion-weighted imaging (PWI), MR angiography (MRA), and conventional MR imaging can be used as the primary imaging technique in ischemic stroke (7–9). The impairment of tissue metabolism and the loss of ion homeostasis are associated with a decline of the apparent diffusion coefficient (ADC), (10) which can be analyzed with stroke MR imaging. We correlated the sites of arterial occlusion in the anterior circulation with initial ADC lesion volumes within 6 hours to long-term infarct growth and size and to reperfusion.
Methods
Patients
We reviewed our prospective MR imaging database of patients with ischemic stroke who were examined within 6 hours after onset from February 2000 to September 2003. We identified 120 patients with vascular occlusion in the anterior circulation, as confirmed with MRA, and completed follow-up imaging on days 5–8. Ninety-five patients received additional follow-up imaging on day 1 after their stroke. We had no limitations regarding other MR findings, age, or severity of symptoms. Multiparametric MR imaging was performed immediately after clinical evaluation and before possible intravenous thrombolysis with tissue-type plasminogen activator. A stroke neurologist (G.T., M.R., J.R.) assessed the patients’ National Institutes of Healthy Stroke Scale (NIHSS) score at each imaging time point. The Barthel index was determined at least 90 days after the stroke. Our local ethics committee approved the study, and informed consent was obtained from all patients.
Imaging Methods
MR imaging was performed with a 1.5-T clinical whole-body unit (Magnetom Symphony/Sonata; Siemens, Erlangen, Germany) with a standard head coil. Among other sequences, imaging included an axial DWI sequence, a PWI sequence, and MRA. Insignificant differences in imaging parameters along the study resulted from hardware or software updates. Recent parameters were the following: a spin-echo echo-planar DWI sequence with a TR/TE of 2600/77, 20 sections, a section thickness of 5 mm, an intersection gap of 1.5 mm, FOV of 230 mm, and original voxel dimensions of 2.4 × 1.8 × 5 mm. ADC was calculated on a pixel-by-pixel basis by using the Stejskal-Tanner-Equation and b values of 0, 500, and 1000 s/mm2 on trace DWIs. Three-dimensional time-of-flight MRA was performed with the following parameters: a magnetization transfer saturation pulse, a TR/TE of 36/6, a flip angle of 25°, three slabs with 32 partitions, a section thickness of 0.8 mm, and an FOV of 150 × 200 mm. PWI was performed with a gradient-echo echo-planar sequence with 18 sections, a TR of 1500 msec, a section thickness of 5 mm, an intersection gap of 1.5 mm, a FOV of 230 mm, and original voxel dimensions of 3.6 × 1.8 × 5 mm. The section position was obtained from DWI and was placed on the center of the DWI lesion. Contrast agent (gadopentetate dimeglumine 25 mL, 0.5 mmol/L; Magnevist, Schering, Germany, or equivalent from other manufacturers) was administered by using a power injector at a rate of 5 mL/s via a flexible cannula (1.2–1.4 mm in diameter) into an antecubital vein. The bolus injection started with the first image and was followed by an infusion of isotonic saline 20 mL at a flow rate of 5 mL/s.
Determination of Occlusion Type and Initial Lesion Volume
The localization of vascular occlusion was determined from the initial MRA study. The type of occlusion was categorized as shown in Figure 1. Postprocessing of the DWI and PWI data were performed offline by using the software SCAN (UCLA Stroke-Attack-Team, Los Angeles, CA). After lesion volumes were manually delineated on ADC maps (ADCman), the lesion volumes with severely decreased ADC were determined in these areas by means of a threshold function (<550 × 10−9mm2/s−1, ADC<550). This threshold was chosen in accordance with the literature (11, 12). The fraction of ADC<550 from ADCman, or ADC<550/ADCman, was then determined.
Types of occlusion were defined on MRA, as follows: a + c was an occlusion of the ICA at the neck accompanied by an MCA embolism, or ICA/MCA occlusion; b, occlusion of the intracranial bifurcation of the ICA, or carotid T occlusion, which included one of 17 cases with an occlusion of the proximal anterior and middle cerebral artery; c, occlusion of the MCA trunk, which included nine of 48 occlusions of the bifurcation or trifurcation lateral to the medial lenticulostriate arteries; and d, occlusion of a single or multiple MCA branch occlusion with free trifurcation, which included three of 36 cases with an additional occlusion of the peripheral anterior cerebral artery.
Determination of Follow-Up Lesion Volume
DWIs obtained with b = 0 s/mm2 on days 5–8 were used as T2-weighted images for the manual delineation of final lesion volumes. The outcome lesion volume was determined by means of manual delineation during CT (n = 11) if MR imaging was not feasible on days 5–8 because of the use of extensive monitoring devices. In an additional analysis of lesion volumes, patients were dichotomized into two age groups: those younger and those at least as old as the mean age. Statistical analysis was performed by using SPSS software (version 11.02; SPSS, Chicago, IL). The nonparametric Mann-Whitney U test was used to evaluate significant differences (P < .05) between types of occlusion for NIHSS scores, Barthel indices, and lesion volumes.
Determination of Recanalization and Reperfusion
Time-to-peak delay maps were calculated from the source PWIs as the time delay from the peak of the arterial input function (AIF) near the contralateral MCA to the maximal changes in signal intensity in the affected hemisphere. A neuroradiologist (T.K.) analyzed recanalization and reperfusion on follow-up MR imaging on day 1 by using both on PWI (time to peak) and MRA studies. The modified Thrombolysis in Myocardial Infarction (TIMI) criteria were used, where TIMI 0 = no recanalization or reperfusion, TIMI 1 = partial recanalization or reperfusion (<20% volume on PWI), TIMI 2 = incomplete recanalization or reperfusion, and TIMI 3 = complete recanalization/reperfusion. TIMI 0 and TIMI 1 were classified as no reperfusion, whereas TIMI 2 and TIMI 3 were defined as reperfusion. For the comparisons of reperfusion rates between the types of occlusion, only data from patients receiving intravenous thrombolytic therapy are presented.
Results
We evaluated 120 patients (41 women, 79 men; age, 62 ± 13 years (mean ± [standard deviation]) who underwent MR imaging within 6 hours (3.0 ± 1.1 hours) after stroke onset. Patients were treated conservatively (with acetylsalicylic acid and enoxaparin, n = 26), with early hemicraniectomy (n = 2), or with intravenous (n = 75) or intra-arterial thrombolysis (n = 7). Ten patients were treated according to the protocol of a randomized, placebo-controlled, double blinded acute stroke trial. Patients had the following types of occlusions: ICA/MCA, n = 19; carotid-T occlusion, n = 17; MCA trunk, n = 48; and MCA branch occlusion, n = 36.
Occlusion Type and Initial Lesion Volume
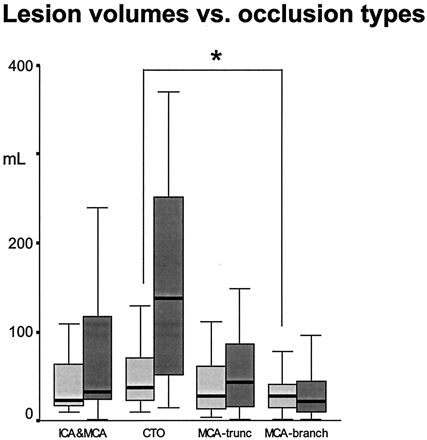
The Table and Figure 2 show the initial ADC lesion and infarct volumes on days 5–8 for each type of occlusion. Paired comparisons of lesion volumes with ADCman by type of occlusion showed significant differences between only carotid-T and MCA branch occlusions (Fig 2). We observed no significant differences between the other occlusion types in multiple pairwise comparisons. The fraction of severe ADC decreases in the entire ADC lesion (ADC<550/ADCman) was lower for all occlusion types compared with branch occlusions; this finding reflected more severe metabolic impairment. Differences between the other occlusion types were not significant. The most severe decreases in initial NIHSS scores were observed in carotid-T occlusions, followed by ICA/MCA and MCA trunk occlusions; patients with branch occlusions were less severely affected.
Boxplots of lesion volumes for ADCman <6 hours after stroke onset and lesion volume on days 5–8 (on T2-weighted or CT images) for each type of occlusion. Asterisk = significant differences in ADCman in multiple pairwise comparisons between types. Infarct volumes were significantly different (P < .05) for all types except for ICA/MCA versus MCA trunk occlusions (MCA-trunc). CTO = carotid-T occlusion.
Patient data
Follow-Up of Lesion Volumes and Outcomes
We observed significant differences in all pairwise comparisons of infarct volumes (at days 5–8) for occlusions of the ICA/MCA (median volume, 32 cm3), carotid T (median volume, 138 cm3), MCA trunk (median volume, 44 cm3), and MCA branch (median volume, 21 cm3). The only exception was the difference between occlusions of the ICA/MCA and MCA trunk. Outcomes were less favorable in proximal vascular occlusions (Table). We found no significant difference in lesion volume after patients were grouped by age (<62 and ≥62 years), either within the entire sample or within the types of occlusion. We observed a trend for larger infarct volumes in carotid-T occlusion among the older patients (P = .079). On the basis of our data, at least 26 patients with carotid-T occlusion would have been required for each age group for us to find a significant difference in infarct volume (P < .05) with a power of 0.80.
Recanalization and Reperfusion
Recanalization was assessed at 24-hour follow-up MR imaging in 65 of 75 patients treated with intravenous thrombolysis. Data in 10 patients could not be analyzed because of movement artifacts or insufficient bolus curves that prevented the correct assessment of the perfusion. Analysis revealed insignificant differences in the rates of reperfusion after occlusion of the MCA branch (71%), MCA trunk (50%), or ICA/MCA (45%), although a significantly decreased rate was observed in carotid-T occlusion (8%). (Fig 3)
Reperfusion assessed on day 1 after stroke onset for each type of occlusion in 65 patients treated with intravenous thrombolysis. CTO = carotid-T occlusion.
Discussion
Our major finding was that ischemic lesions may be initially small, even in patients with proximal vascular occlusions, as lesions may subsequently grow in this group (Figs 2 and 4). In fact, patients with carotid-T occlusions had significantly larger initial ADC lesions only when compared with patients with MCA branch occlusions (Fig 2). However, differences in lesion volumes between the types of occlusion on days 5–8 were substantially larger. We observed significant differences between each infarct volume, resulting in an increase from distal to proximal (Fig 2); only infarct volumes MCA trunk and ICA/MCA occlusions were not different. We hypothesized that the low recanalization rates in several patients with carotid-T occlusion—and not necessarily the early metabolic affect—is the main cause of large infarct volumes (Fig 3). This finding supports the hypothesis that the tissue prognosis is not entirely determined at time of early imaging (<6 hours).
T2-weighted (b = 0 s/mm2, top) and ADC (bottom) images obtained in patients with proximal vascular occlusions show considerable lesion growth without recanalization. Left box, Images obtained in a 71-year-old man with an ICA/MCA occlusion treated with intravenous thrombolysis. Right box, Images in a 67-year-old woman with a carotid-T occlusion treated with craniotomy.
Our data support the importance of the MRA in the stroke MR imaging protocol in this regard: In contrast to perfusion imaging, MRA directly reveals the location of the vascular occlusion and subsequently the probability of vessel recanalization. The size of the thrombus, which is related to the site of vascular occlusion on MRA, might be an important determinant of vascular recanalization rates and cannot be derived from DWI or PWI alone. More generally, our findings may also support the value of other methods of vascular imaging. CT angiography in a CT-based stroke protocol might even be superior to time-of-flight MRA in the evaluation of collateral flow and of low flow distal to occlusions (13). In previous MR imaging-based studies, recanalization rates were higher in distal occlusions than in proximal occlusions (14) and in the MCA versus the ICA (15). Some groups have investigated the relation of the vascular patency on MRA to outcomes (16–18). Rordorf et al (16) observed that patients with proximal occlusions have further lesion growth, whereas patients with distal branch occlusions, not visible on MRA, did not. Because of differences in focus and because of the smaller number of patients, the investigators did not discriminate between more detailed types of occlusion and their specific ischemic lesion.
Vascular occlusion, as determined on angiography, is a substantially better predictor of poor outcome in patients undergoing intra-arterial thrombolytic therapy than parenchymal signs of ischemia, as determined on CT (3, 19). These observations underline the need for more sensitive techniques for assessing the ischemic lesion on a parenchymal level. We chose ADC values as measures of the initial ischemic impairment. Despite some lack of understanding about the biophysical background of decreased ADC, the failure of ion pumps and depolarization of the anoxic cell membrane corresponds well with the evolution of decreases in ADC (10, 20). Low ADC values are found in the ischemic core with severely decrease cerebral blood flow and depletion of adenosine triphosphate at 2 hours after vascular occlusion, but ADC in penumbral tissue was moderately decreased (21). We observed a lower fraction of severe decreases in ADC (ADC<550/ADCman) in occlusions of the MCA branch; this finding reflected less severe metabolic impairment compared with that of the other types of occlusion. In contrast, patients with proximal vascular occlusions probably had a breakdown of collateralization and tissue damage in the terminal branches, especially in the striatocapsular region.
The anatomic variability of the cerebral vascular system, and consequently the variable extent of the cerebral vascular territories, is well described (22): One type of vascular occlusion may result in different volumes and degrees of ischemia because the collateral circulation is highly variable. Accordingly, we observed considerable heterogeneity in initial ADC lesion volumes within each type of occlusion (Fig 5, Table). We observed only small differences in ADCman in occlusions of the ICA/MCA, carotid T, MCA trunk, and MCA branches, with median values of 23–37 cm3 (Table). Therefore, the initial ADC lesion could be small, even in patients with carotid-T occlusion or MCA trunk occlusion. In contrast, differences in infarct volumes on day 5–8 were large, decreasing from proximal (138 cm3 in carotid T occlusions) to distal (21 cm3 in MCA branch occlusions). This pattern may be explained by sufficient collateralization at time of imaging, but while ischemia persisted, the tissue evolved into infarction. We believe it is safe to conclude that delayed breakdown of collateral pathways causes the growth of the lesion, and timely recanalization might save considerable tissue volume, even in cases of carotid-T occlusions.
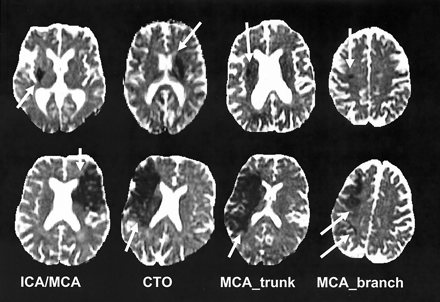
Smallest (top row) and largest (bottom row) ADC lesions <6 hours after stroke onset are depicted for each type of occlusion (arrow). Section showing the maximal extent of the lesion was chosen in each case. CTO = carotid-T occlusion.
We observed no difference in the initial ischemic ADC lesion for combined ICA/MCA and carotid-T occlusions versus occlusions of MCA trunk, and we found no difference in final infarct volume between ICA/MCA occlusions and occlusions of the MCA trunk. Several reasons may be responsible for the comparably small infarctions in the ICA/MCA group: First, ICA/MCA occlusions result from arterioarterial embolisms originating in ICA stenosis or occlusion at the extracranial bifurcation. Although unproven, chronic but compensated ischemia may have led to improved leptomeningeal collateralization in patients with long standing ICA stenosis. Second, the circle of Willis provides flow via the anterior communicating artery in as many as 88% of all cases (23) after prompt recanalization of the MCA occlusion. Third, cellular adaptation called ischemic preconditioning might have occurred (24).
At follow-up, differences in infarct volumes between carotid-T and ICA/MCA occlusions might also be explained by differences in reperfusion rates (Fig 3). We observed the lowest recanalization rate in carotid-T occlusions (8%) and the highest rate in MCA branch occlusions (71%). Our recanalization rates must be interpreted cautiously because of the relatively small samples in each group. Nevertheless, our data are comparable to those of previous angiographic studies (4–6). Several groups described rates of recanalization after intra-arterial thrombolysis: 63% for ICA/MCA occlusion at M1 and M2 (6), 70–79% for MCA trunk occlusion (5, 6), and 55–64% for MCA branch occlusion (5, 6). In line with our results, the worst recanalization rates, 23–28%, are described for the carotid-T occlusion (4, 6), which is known to be predictive of fatal brain swelling (19). The initial ADC lesions were small in several patients with carotid-T occlusion probably due to good initial collateralization (3). Nevertheless, without recanalization, these patients had large infarctions.
As others reported (19, 25), we had some patients with carotid-T occlusion who had a good outcome (Table). However, intravenous thrombolysis seemed insufficient for the treatment of carotid-T occlusion, as sufficient reperfusion was observed in only one of our 13 patients. Possible approaches to improving the results of recanalization may be more rapid clot lysis and fragmentation or retrieval by using mechanical devices or use of intravenous tissue plasminogen activator with sonography or a drug such as abciximab (26). Some have hypothesized that a combination of approaches might improve clinical outcomes of patients with large clots (27).
Conclusion
Early ischemic lesions may be small, even in patients with proximal-vascular occlusions. In the presence of low recanalization rates, most patients with carotid-T occlusion have considerable lesion growth and poor outcomes. This lesion growth might be averted with improved early recanalization of proximal vascular occlusions.
Footnotes
Presented in part at the Annual Meeting of the American Society of Neuroradiology, Seattle, WA, June 5–11, 2004.
References
- Received July 30, 2004.
- Accepted after revision September 24, 2004.
- Copyright © American Society of Neuroradiology