Abstract
BACKGROUND AND PURPOSE: Angioplasty and stent placement have been reported for the treatment of intracranial stenosis. This study was undertaken to assess the efficacy and long-term clinical outcome of angioplasty without stent placement for patients with symptomatic intracranial stenosis.
METHODS: A retrospective study was done to evaluate 36 patients with 37 symptomatic atherosclerotic intracranial stenosis who underwent primary balloon angioplasty. All patients had symptoms despite medical therapy. Thirty-four patients were available for follow-up ranging from 6 to 128 months. Mean follow-up was 52.9 months.
RESULTS: Mean pretreatment stenosis was 84.2% before angioplasty and 43.3% after angioplasty. The periprocedural death and stroke rate was 8.3% (two deaths and one minor stroke). Two patients had strokes in the territory of angioplasty at 2 and 37 months after angioplasty. The annual stroke rate in the territory appropriate to the site of angioplasty was 3.36%, and for those patients with a residual stenosis of ≥50% it was 4.5%. Patients with iatrogenic dissection (n = 11) did not have transient ischemic attacks or strokes after treatment.
CONCLUSION: Results of long-term follow-up suggest that intracranial angioplasty without stent placement reduces the risk of further stroke in symptomatic patients.
Endovascular therapy for patients with symptomatic intracranial atherosclerotic stenosis is becoming more widespread. Primary angioplasty has been reported in a number of series, although these series had relatively brief clinical follow-up (1–5). More recently, primary intracranial stent placement has been suggested as an alternative to angioplasty alone (6, 7). Proponents of intracranial stent placement have suggested that outcomes and complications, such as dissection, may be improved with this method compared with angioplasty alone.
This study was undertaken to evaluate the long-term outcome of angioplasty alone in the treatment of symptomatic intracranial stenoses. We specifically assessed the incidence of ischemic stroke in the territory of treatment, as well as the incidence of all strokes and death, although this was not a study of angiographic follow-up. We also assessed long-term outcomes after angioplasty-induced dissection and when a significant (≥50%) residual stenosis is seen after angioplasty.
Methods
We retrospectively evaluated 36 consecutive patients with 37 symptomatic atherosclerotic intracranial stenoses who underwent primary balloon angioplasty at our institution between 1992 and 2002. All patients had symptomatic intracranial stenoses despite antithrombotic therapy and then underwent elective angioplasty. Intracranial lesions were defined as those at or above the precavernous or upper petrous carotid artery in the anterior circulation and above the C1 portion of the distal vertebral artery in the posterior circulation. Patients had to have a minimum of 6 months of clinical follow-up. Patients undergoing intracranial angioplasty in the setting of acute stroke were excluded (approximately 10 patients in the study period). Those with intracranial lesions believed to be due to etiologies other than atherosclerosis (e.g., vasospasm) were also excluded (approximately 30 patients). In addition, patients with tandem intracranial and extracranial disease undergoing angioplasty at both sites were excluded (approximately five patients). Therefore, this study did not include patients with hemodynamically significant extracranial cerebrovascular disease at the time of angioplasty.
A stroke neurologist (D.C.T., G.W.A.) evaluated all patients, who were determined to have had a prior transient ischemic attack (TIA) or stroke in a territory appropriate to lesions while receiving antiplatelet or anticoagulant therapy. Sixteen patients were receiving anticoagulant therapy (warfarin) alone. Twelve patients were receiving combined antiplatelet therapy (aspirin, ticlopidine, or clopidogrel) and anticoagulant therapy (warfarin or heparin), and eight were receiving antiplatelet therapy alone at the time of their last symptomatic event.
Patients were treated with a variety of angioplasty balloons varying from 2 × 10 to 4 × 20 mm. These were introduced through 6F or 7F guide catheters. Balloon sizes were chosen on the basis of angiographically obtained measurements. The balloons were deliberately undersized by about 0.5 mm (as they are generally sized in 0.5 mm increments) to reduce the risk of vessel rupture. The shortest length needed to cross the lesion was chosen to minimize any problems with the tracking of the balloon. Catheterizations were done through the femoral artery with introduction of the guide catheter into the high cervical segment of the carotid or vertebral artery. Balloons were introduced over a 0.014-in guidewire that was used to cross the lesion. Angioplasty balloons were gradually inflated over several seconds to the nominal inflation pressures recommended by the manufacturer and kept inflated at this pressure for 30–45 seconds. In general, even a mild degree of improvement in luminal stenosis was considered acceptable. Balloons were therefore inflated once or at most two times. In addition, balloons were not generally upsized.
In advance of the procedure, patients were given an antiplatelet agent (aspirin 325 mg daily, ticlopidine 250 mg twice daily, or clopidogrel 75 mg daily) if they were not already receiving it. If patients were taking coumadin as outpatient therapy, this was generally discontinued 5 days before the procedure so that the international normalized ratio was <1.5 on the day of the procedure. Before angioplasty, patients received systemic anticoagulation with intravenous heparin. After angioplasty, if the residual stenosis was <50% and the vessel wall was smooth, anticoagulation was discontinued. When the postangioplasty angiogram showed >50% stenosis or when the vessel showed evidence of intimal tear, anticoagulation was continued for at least 8–12 weeks after angioplasty. In addition, antiplatelet medications (aspirin, clopidogrel, or the combination of aspirin/extended-release dipyridamole) were give long term: 23 patients received coumadin for the 8–12-week period, 16 patients were given coumadin alone long term, and 11 patients given aspirin alone (four, clopidogrel; one, aspirin/extended-release dipyridamole; one, ticlopidine; and one, coumadin and ticlopidine).
Stenosis was measured by using a previously described technique shown to have good interobserver and intraobserver reliability (8). This measurement was based on the ratio of the narrowest diameter on any projection to that of the portion of the artery nearest the stenosis that is judged to be normal. The proximal portion of the artery is the first choice for the normal segment. However, if the stenosis involved the proximal portion of the artery or if evidence suggested significant atherosclerotic disease, the distal segment of the artery beyond the stenosis was used. These measurements were generally done by using the software available for stenosis measurement provided on the digital angiography equipment used for the procedures. Stenoses measurements were rounded to the nearest 5%. In cases where extreme preocclusive stenosis made accurate measurement of the degree of stenosis too difficult, the stenosis was estimated to be 95%.
Follow-up was performed by means of telephone contact by a nurse coordinator (M.L.M.). Patients were asked about hospitalizations, changes in medication, new neurologic events, and new or transient neurologic deficits after the angioplasty procedure. The annual stroke rate was the ratio of stroke rate to follow-up time. For calculation purposes, stroke rate was assumed to be binomially distributed, and follow-up time, normally distributed. The variance of the annual stroke rate was then calculated by using a formula that approximates the variance of a ratio given the expected values and variances of the numerator and denominator (9). Using the variance for the annual stroke rate ratio, we calculated a confidence interval for the mean annual stroke rate, assuming a normal distribution.
Results
Table 1 shows the clinical and demographic characteristics for the study group, including the known risk factors for stroke in this group. The treatment group included 27 men and nine women aged 31–84 years (mean, 62.2 years). Table 2 shows the locations of their lesions.
Clinical and demographic characteristics
Lesion locations
Sixteen patients had lesions in the anterior circulation, and 21 patients had lesions in the posterior circulation. In one patient with stenoses of both vertebral arteries, both lesions were treated. Before treatment, stenoses varied between 60% and 95% (mean ± SD, 84.2% ± 10.7%). In all cases, the guidewire crossed the lesion, and the angioplasty balloon was inflated. In 35 patients, successful angioplasty was done with a change in the morphology of the lesion and improvement in the degree of stenosis seen on the postangioplasty angiogram. In a patient with stenosis of the middle cerebral artery (MCA), vessel rupture occurred at the time of angioplasty, and a postangioplasty stenosis measurement was not available. Residual stenosis on the immediate posttreatment angiogram varied between 5% and 75% (43.3% ± 18.9%). Even a mild degree of improvement in luminal stenosis was considered acceptable, and 18 of 36 lesions had residual stenoses of 50% or greater. These posttreatment stenoses varied from 50% to 90% (56.5% ± 8.3%).
Three clinical complications (8.3%) occurred within 30 days of angioplasty, resulting in stroke (one patient) or death (two patients). In one case, rupture of the MCA resulted in patient death at the time of the procedure. A second death occurred approximately 1 day after MCA angioplasty, with the development of a large intraparenchymal hematoma thought to be due to a reperfusion hemorrhage. One patient had a minor ischemic stroke with a focal lacuna in the brainstem and made a full recovery. There were no major ischemic strokes in the periprocedural period.
Clinical follow-up was done in all 34 patients available for follow-up, which varied from 6 to 128 months (52.9 ± 38.4 months). Twenty-nine patients had follow-up >24 months. Total aggregate follow-up yielded 154.4 patient-years. In the follow-up period, two strokes occurred in a territory appropriate to the site of angioplasty beyond 30 days. One occurred at 2 months after angioplasty, and 1, at 37 months. The former occurred in a patient who had discontinued his anticoagulant and antiplatelet therapy on his own. Three strokes occurred in the study group outside of the territory of angioplasty at 32 months, 36 months, and 9 years. We noted three deaths: one at 25 months (myocardial infarct), one at 40 months (cardiomyopathy and respiratory arrest), and one at 10 years (myocardial infarct).
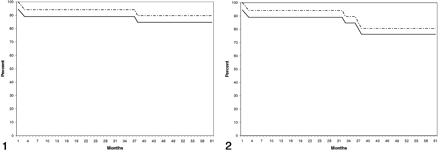
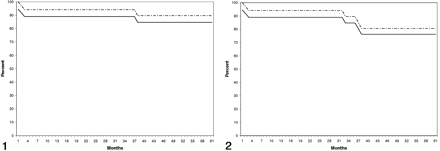
The annual rate for strokes in the territory of treatment including the three periprocedural events was 3.36% (95% confidence interval: 1.15, 3.37). The annual stroke rate for all strokes regardless of the territory was 5.38% (95% confidence interval: 2.00, 8.75). On Kaplan-Meier analysis, stroke-free survival at 5 years for strokes appropriate to the territory of angioplasty was 84.7%, including the periprocedural complications (Fig 1). Stroke-free survival for all strokes was 76.25%, including the periprocedural complications (Fig 2).
Stroke-free Kaplan-Meier survival plot shows the percentage of patients alive without stroke in the territory appropriate to the site of angioplasty. Solid line represents the outcome for all treated patients; dashed line, stroke-free survival excluding strokes as procedural complications.
Stroke-free Kaplan-Meier survival plot shows the percentage of patients alive without stroke after angioplasty. Solid line represents the outcome for all treated patients; dashed line, stroke-free survival excluding strokes as procedural complications.
Routine imaging follow-up was not performed at our center during this retrospective study. Vascular imaging follow-up was done in 20 patients (MR angiography in six, angiography in 14). Subtle changes in the degree of stenosis were not thought to be discernable with MR angiography. However, all of these patients had patent vessels in the area of stenosis, and no definite restenosis was seen. Fourteen patients underwent angiographic follow-up 2 months to 9 years after angioplasty. In nine of these patients, their condition was judged stable, four had mild improvement in the degree of stenosis, and one patient had an asymptomatic occlusion.
We also examined stroke rates in the subset of patients with ≥50% residual stenosis on the postangioplasty angiogram and in patients with an intimal flap on the postangioplasty angiogram that indicated a dissection. Eighteen patients had ≥50% stenosis after angioplasty; follow-up was available on 17 (7–128 months; mean, 49.3 months). This group included one patient with a periprocedural event and one with stroke at 2 months after angioplasty. No other strokes occurred in this group. The annual stroke rate in patients was 4.5%, including the periprocedural event. In 11 patients, the postangioplasty angiogram showed evidence of a dissection, as manifest by the presence of an intimal tear. No strokes or TIAs were observed in this group on follow-up.
Discussion
We report the results of treating 36 patients with symptomatic intracranial stenosis by using angioplasty alone. Thirty-four patients were followed for a mean of approximately 4.5 years. The annual rate for strokes in the territory of treatment was 3.36%, and the annual rate for all strokes regardless of territory was 5.38%. These results suggest that this treatment provides a durable clinical result at long-term follow-up.
Several prior groups have suggested that intracranial angioplasty can be performed with a high degree of technical success. However, the studies have often lacked long-term outcome data describing how the procedure affects the stroke rate. Connors and Wojack (1) reported their multiyear experience in the treatment of 70 patients. On the basis of an evolution in their treatment technique, they divided their data into three periods of treatment. The most recent period in 50 patients demonstrated good angiographic and short-term clinical outcomes in 49 (with one patient dying from vessel perforation). Because of the retrospective nature of their study, the fact that several institutions were involved in performing the procedures, and the wide geographic locations of their patients, they were unable to obtain follow-up data. Gress et al (2) describe a single-center experience by using angioplasty to treat symptomatic vertebrobasilar ischemia in 25 patients. They reported an overall 28% procedural risk of stroke or death and a 16% risk of disabling stroke or death, but they did not include long-term follow-up data for patients who were successfully treated. Mori et al (3) reported on the treatment of 42 patients, 32 of whom underwent successful angioplasty. However, a cumulative risk for stroke at 1 and 2 years was reported for all 42 patients; this makes the true rate of stroke in patients with successful angioplasty difficult to ascertain. Clark et al (4) followed up 17 patients for 8–54 months (mean, 22 ± l6 months) and found no neurologic events in the territories of the angioplasty vessels. Marks et al (5) reported the results of angioplasty in 23 patients treated over 5 years and followed up for 16–74 months (mean, 35.4 months). The authors found an annual stroke rate of 3.2% for strokes in the territory appropriate to the angioplasty site.
Available natural history data suggests that angioplasty notably affects the rate of stroke by in this group of patients. The EC-IC Bypass Study Group (10) provided prospective data on the outcome of patients with symptomatic atherosclerotic disease in the carotid circulation. A subgroup of 352 study participants had atherosclerotic disease of the MCA and participants were randomized to receive bypass surgery versus medical treatment (which consisted of aspirin 325 mg four times a day). In the medical treatment arm, 164 patients were followed up for a mean of 42 months. Patients who had MCA stenoses had an ipsilateral stroke rate of 7.8% and an overall annual stroke rate of 9.5%. Prospective data are not available for patients with posterior circulation intracranial stenoses. Retrospective data from the Warfarin-Aspirin Symptomatic Intracranial Disease Study (WASID) (11) are available. This study included 68 symptomatic patients with >50% stenosis of the major posterior circulation arteries who were followed up for a mean time of 13.8 months. About 87% of these patients had vertebral or basilar artery stenosis. They had a 7.8% annual rate of strokes appropriate to the symptomatic stenosed artery for vertebral artery disease and a 10.7% annual rate for basilar artery disease. Another study has documented the stroke rate in 102 patients with symptomatic intracranial vertebrobasilar stenosis followed up for a mean of 15 ± 15.9 months (12). These patients had a 10.9% annual recurrent stroke rate and 24.2% rate of recurrent stroke and death rate.
Our patients may have had a poorer natural history, as antithrombotic therapy had already failed. One retrospective study has shown the outcome in patients with symptomatic intracranial stenosis in whom antithrombotic therapy failed (13): 29 patients had a TIA or stroke while receiving anti-thrombotic therapy (antiplatelet agents or anticoagulants). Fifteen (51.7%) had a new TIA (n = 7), had a stroke (n = 7), or died (n = 1) at a median time of 36 days after their initial event. This finding suggests an extremely high rate of recurrent symptomatic events and a short time to failure when antithrombotic therapy fails.
Small series of patients with intracranial stent placement in the same setting have been reported, with mean follow-up times of less than a year (6, 7). Potential advantages for stent placement (rather than angioplasty alone) include a decreased risk of closure due to dissection and improved long-term patency rates from larger luminal diameters after the procedure (14). We examined stroke rates in a subset of patients with ≥50% residual stenosis on postangioplasty angiograms. In this subgroup of 18 patients with a mean follow-up time of >4 years, the annual stroke rate in the territory appropriate to the site of angioplasty remained low at only 3%.
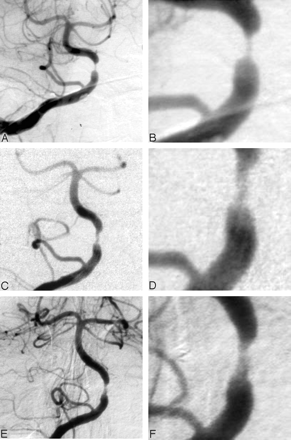
A number of explanations may account for the reduced incidence of strokes, even in patients with residual stenoses of >50% at the completion angioplasty. The main goal of the angioplasty is to improve blood flow. Some of these lesions may manifest symptoms only after they become extremely tight, and even relatively minor corrections in the degree of stenosis may improve symptoms and outcomes. Flow is proportional to the fourth power of the radius of the vessel. This means that the flow is approximately doubled when the radius is increased by 20% or the diameter by 10%, so that small increases in the luminal diameter may result in large increases in the flow. Another possible explanation for this observation is that remodeling may occur in the stenotic region as the angioplasty site heals. We did not routinely perform angiographic follow-up on our patients; however, in some cases (especially earlier in our experience), we did have angiographic follow-up and observed a mild reduction in the severity of residual stenosis or remodeling that occurs (Fig 3). We also evaluated outcomes in the subset of 11 patients whose postangioplasty angiograms showed evidence of dissection. Importantly, no strokes or TIAs occurred in this group during follow-up.
This 61-year-old woman had repeated vertebral basilar TIAs while receiving warfarin.
A and B, Anterior pretreatment right vertebral artery angiograms (magnified image in D) show severe stenosis of the proximal basilar artery.
C and D, Anterior right vertebral artery angiograms obtained immediately after angioplasty show improvement in the stenosis but continued moderate luminal narrowing.
E and F, Angiograms obtained 2 months after angioplasty show mild improvement in the stenosis with remodeling. At last follow-up (60 months), the patient did not have additional symptoms.
Conclusion
This report documents the long-term follow-up findings (stroke and death rates at a mean follow-up time of over 4 years) in patients with symptomatic intracranial stenosis who underwent angioplasty without stent placement. We found a low rate of stroke recurrence, which suggests that this procedure has a durable effect. These data compare favorably with reported stroke recurrence rates in medical patients with symptomatic lesions. In addition, the data do not appear to be substantially different for the subset of patients with ≥50% residual stenosis after angioplasty.
References
- Received February 23, 2004.
- Accepted after revision July 16, 2004.
- Copyright © American Society of Neuroradiology