Abstract
BACKGROUND AND PURPOSE: Contrast staining is a common finding after endovascular treatment of acute ischemic stroke. It typically occurs in infarcted tissue and is considered an indicator of irreversible brain damage. Contrast staining in noninfarcted tissue has not been systematically investigated. We sought to assess the incidence, risk factors, and clinical significance of contrast staining in noninfarcted tissue after endovascular treatment.
MATERIALS AND METHODS: We conducted a retrospective review of consecutive patients who underwent endovascular treatment for anterior circulation large-vessel occlusion acute ischemic stroke. Contrast staining, defined as new hyperdensity on CT after endovascular treatment, was categorized as either contrast staining in infarcted tissue if the stained region demonstrated restricted diffusion on follow-up MR imaging or contrast staining in noninfarcted tissue if the stained region demonstrated no restricted diffusion. Baseline differences between patients with and without contrast staining in noninfarcted tissue were compared. Logistic regression was used to identify independent associations for contrast staining in noninfarcted tissue after endovascular treatment.
RESULTS: Among 194 patients who underwent endovascular treatment for large-vessel occlusion acute ischemic stroke and met the inclusion criteria, contrast staining in infarcted tissue was noted in 52/194 (26.8%) patients; contrast staining in noninfarcted tissue, in 26 (13.4%) patients. Both contrast staining in infarcted tissue and contrast staining in noninfarcted tissue were noted in 5.6% (11/194). Patients with contrast staining in noninfarcted tissue were found to have a higher likelihood of having an ASPECTS of 8–10, to be associated with contrast staining in infarcted tissue, and to achieve successful reperfusion compared with those without contrast staining in noninfarcted tissue. In contrast staining in noninfarcted tissue regions, the average attenuation was 40 HU, significantly lower than the contrast staining in infarcted tissue regions (53 HU). None of the patients with contrast staining in noninfarcted tissue had clinical worsening during their hospital stay. The median discharge mRS was significantly lower in patients with contrast staining in noninfarcted tissue than in those without (3 versus 4; P = .018). No independent predictors of contrast staining in noninfarcted tissue were found.
CONCLUSIONS: Contrast staining can be seen outside the infarcted tissue after endovascular treatment of acute ischemic stroke, likely attributable to the reversible disruption of the BBB in ischemic but not infarcted tissue. While generally benign, understanding its characteristics is important because it may mimic pathologic conditions such as infarcted tissue and cerebral edema.
ABBREVIATIONS:
- AIS
- acute ischemic stroke
- CS
- contrast staining
- CS-I
- contrast staining in infarcted tissue
- CS-NI
- contrast staining in noninfarcted tissue
- EVT
- endovascular treatment
- HU
- Hounsfield unit
- IQR
- interquartile range
- LVO
- large-vessel occlusion
Accurate interpretation of the imaging findings after endovascular therapy (EVT) for acute ischemic stroke (AIS) is important for appropriate management and prognostication. Hyperdensities on NCCT head scans are common findings after EVT, having been described in up to 84% of patients.1⇓⇓⇓-5 Although these hyperdensities may represent extravasated blood products or other hemorrhagic complications, most are due to retained iodinated contrast material from intra-arterial iodine contrast injection, a phenomenon termed contrast staining (CS).
Brain ischemia is known to induce gradual and time-dependent changes in the integrity of the BBB. which is impermeable to contrast molecules in a physiologic state.6⇓-8 Varying degrees of increased permeability permit the fluid and plasma protein leakage seen in cerebral edema, contrast material leakage causing CS, and the blood product extravasation seen in hemorrhagic transformation.6,7,9 The increased duration and severity of the ischemic insult are associated with a higher degree of microvascular injury and BBB disruption, while collateral circulation in the involved region may protect the microvasculature.6,10,11
The presence of CS has been considered an indicator of irreversible brain injury and has been associated with the development of symptomatic intracranial hemorrhage and poor clinical outcome.12⇓⇓-15 However, CS may also occur in noninfarcted territories and may resolve without sequelae. This phenomenon of transient CS in noninfarcted tissue poses radiologic and management challenges because it may mimic pathologic conditions such as infarcted tissue, extravasated blood products, intracranial hemorrhage, and cerebral edema. Prior studies that demonstrated the association of CS with irreversible injury focused on staining in areas of core infarct, and, to our knowledge, CS in the noninfarcted tissue has not been systematically investigated. This study aimed to characterize the incidence of CS in noninfarted tissue (CS-NI), its radiologic characteristics, potential risk factors, and its clinical and imaging outcomes.
MATERIALS AND METHODS
Ethics approval was obtained from Boston University institutional review board. According to institutional protocol, written informed consent for EVT was obtained from patients and/or their legal representatives. Anonymized data are available from the corresponding author on reasonable request. This article followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Study Design and Patient Selection
We performed a retrospective review of a prospectively maintained neuroendovascular database of consecutive patients who presented with anterior circulation large-vessel occlusion AIS (LVO-AIS) and underwent EVT between January 2016 and January 2022. Patients were included in the analysis under the following conditions: 1) an NCCT was obtained within 24 hours after EVT, 2) a follow-up MR imaging of the brain was obtained within 72 hours after EVT, and 3) clinical follow-up was available 3 months after EVT. Patients were excluded if they had posterior circulation stroke, complete infarction of the affected vascular territory, and subarachnoid or parenchymal hemorrhage, as determined by SWI.
Clinical, Radiologic, and Endovascular Data Collection
Patient demographics (age, sex), vascular risk factors (diabetes mellitus, hypertension, atrial fibrillation, coronary artery disease, kidney disease, heart failure, smoking), preprocedural stroke and imaging characteristics, procedural details, follow-up imaging data, and outcome data are presented as detailed in the Online Supplemental Data and Tables 1 and 2. In addition, blood pressure measurements at presentation and intraprocedurally, NIHSS shift at 24 hours after the procedure, NIHSS at 1 week or discharge if earlier, and the mRS at 90 days after EVT were recorded. Clinical worsening postprocedure was defined as neurologic deterioration of ≥ 4points on the NIHSS from baseline.
Clinical outcomes by contrast staining status
Crude and adjusted odds of contrast staining outside the infarcted tissuea
Imaging Analysis
All NCCTs within 24 hours after EVT and follow-up MR imaging within 72 hours after EVT were independently evaluated by 2 board-certified neuroradiologists with 5 (M.A,) and 15 (B.N.S.) years of experience who were blinded to treatment details. CS was defined as new parenchymal hyperdensities on early CT imaging performed within 24 hours after EVT without evidence of blood products on SWI. CS was categorized as contrast staining in infarcted tissue (CS-I) if the stained region demonstrated restricted diffusion on follow-up DWI. CS was categorized as CS-NI if the stained region demonstrated no corresponding signal abnormality on T2, FLAIR, or DWI.
For patients with CS, the Hounsfield unit (HU) of the attenuation of the stained tissue was measured using the free-form ROI and compared with the contralateral symmetric normal region. CT and MR imaging were also analyzed for associated signs of infarction (loss of the gray-white matter, gyral swelling, and sulcal effacement) and signs of intracranial hemorrhage.
Statistical Analysis
The descriptive analysis compared demographics, medical comorbidities, radiographic details, and procedural details between patients with and without CS-NI. Continuous variables were expressed as mean (SD) and median (interquartile range [IQR]) and were compared using the Student t test or Mann-Whitney U test, as appropriate. Differences in categoric variables were examined with the χ2 test or Fisher exact test. Variables achieving P ≤ .1 in univariable analyses and a priori selected variables based on prior literature (eg, hypertension and diabetes mellitus) were carried forward into a multivariable logistic regression to evaluate potential independent factors associated with CS-NI. To account for sparse data, all logistic regression models used the Firth adjustment.16 All statistical analyses were performed on SAS, Version 9.4 (SAS Institute). All tests were 2-sided, and a P value < .05 was considered significant.
RESULTS
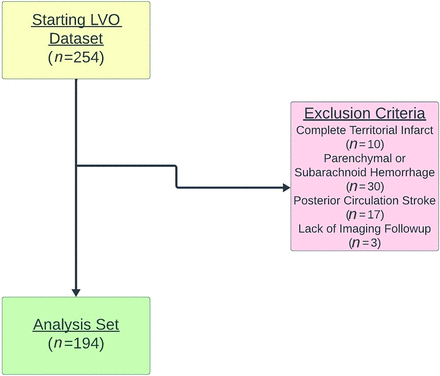
Among 254 patients who underwent EVT for LVO-AIS during the study period, 194 patients were included after the exclusion of 60 patients due to complete territorial infarct (n = 10), parenchymal or subarachnoid hemorrhage (n = 30), posterior circulation stroke (n = 17), or lack of imaging follow-up (n = 3) (Fig 1).
Eligibility criteria for study inclusion are demonstrated.
Among the 194 included patients, CS-I was noted in 52 (26.8%) patients, and CS-NI was noted in 26 (13.4%) patients. Both CS-I and CS-NI were noted in 5.6% (11/194) of patients who underwent EVT in our study. Examples of CS-NI are depicted in Figs 2 and 3. In the overall cohort, the median age was 72 (IQR, 59–82) years, 48.5% were men, and the median baseline NIHSS was 18 (IQR, 13–21), with no difference between groups. Other baseline demographic and clinical characteristics of patients with or without CS-NI are shown in the Online Supplemental Data.
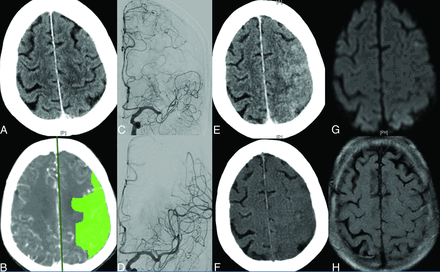
Noncontrast head CT (A) and perfusion imaging map (B) of a patient who presented with an acute left M1 occlusion stroke, showing preserved brain parenchyma and a large area of penumbra (in green, B). Angiogram before (C) and after (D) mechanical thrombectomy shows complete recanalization of the left MCA territory. Note good leptomeningeal collaterals in the contrast-stained region. NCCT performed after the procedure (E) shows contrast staining with increased attenuation and sulcal effacement in the left cerebral convexity, which was completely effaced on the virtual noncontrast dual-energy CT (F). MR imaging follow-up shows normal findings on MR images with no corresponding signal abnormality on DWI (G) and FLAIR (H) images.
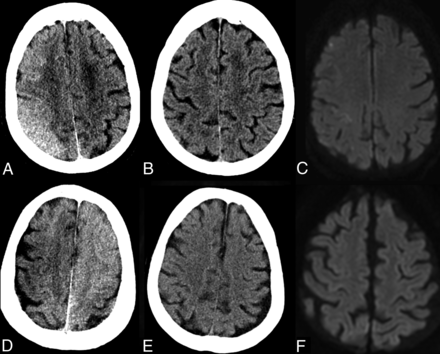
Transient contrast staining of noninfarcted regions in the right cerebral convexity after endovascular treatment of a right M1 MCA occlusion (A–C) and left M1 MCA occlusion (D–F), manifesting as increased parenchymal attenuation and effacement of the cortical sulci (A and D) on the early posttreatment CT imaging. Repeat CT imaging 48–72 hours later (B and E) shows normalization of the brain parenchyma and cortical sulci. DWI (C and F) shows normal brain parenchyma with no restricted diffusion in the previously stained region.
Imaging and Procedural Characteristics
The most common occlusion site was the M1 segment of the MCA (45.9%, 89/194). There was no difference in lesion location between those with and without CS-NI (P = .21). The most common first-line technique of EVT was a stent retriever (74.7%, 145/194), and a balloon guide catheter was used in 78.9% (153/194) of cases. In 84.5% (164/194) of cases, ≤3 passes were completed. Patients with successful reperfusion, defined as an expanded TICI of ≥2b, were more likely to develop CS-NI than those without successful reperfusion (16.2% versus 0, P = .01). Patients with ASPECTS of 8–10 on preprocedural CT were also more likely to have CS-NI than those with ASPECTS of 5–7 (16.2% versus 5.8%, P = .059); however, this difference did not reach statistical significance. There was no difference between the median volume of contrast used during the procedure, the mean systolic blood pressure, or blood pressure variability between those with and without CS-NI. There was no statistical significance in the duration of the procedure between patients with or without CS-NI. There was a higher incidence of CS-NI observed in patients with kidney disease; however, the increase did not reach statistical significance.
NCCT was performed at a median interval of 517 (IQR, 400–767) minutes after EVT, with no difference between those with and without CS-NI. In CS-NI regions, the average mean attenuation was 40 HU, and the average maximum attenuation was 53 HU, which was greater than the average mean (32 HU) and average maximum attenuation (43 HU) in the contralateral normal hemisphere (P < .001). For patients with both CS-NI and CS-I (11 patients), HU values were higher in CS-I regions than in CS-NI regions for both the average mean (53 versus 40; P = .027) and the average maximum (70 versus 52; P = .014). In Hounsfield unit measurements in areas considered normal, CS-I or CS-NI were calculated and are shown in Table 3. While there was a trend suggesting a higher incidence of CS-NI in patients with CS-I, CS-NI was identified in 22.2% (11/52) of patients with CS-I compared with 10.6% (15/142) in patients without CS-I; this difference did not reach statistical significance. In our cohort, only 8 of the 194 patients underwent CT perfusion, and 2 of them exhibited CS-NI.
Hounsfield units in healthy controls, penumbra, and core tissuea
Outcomes
None of the patients with CS-NI had clinical worsening after EVT compared with 12.5% of patients with CS-I (0% versus 12.5%; P = .08). There was no difference in the median NIHSS at 24 hours post-EVT (12 versus 13; P = .77) or median NIHSS at discharge (6 versus 9.5; P = .08) between patients with and without CS-NI. The median discharge mRS was significantly lower in patients with CS-NI compared with those without (3 versus 4; P = .018), and there was no difference in median 3-month mRS between the 2 groups (3 versus 3; P = .06) (Table 1).
Univariable and Multivariable Analysis
In univariable analysis, no significant differences between patients with and without CS-NI were observed by sex, comorbidities, NIHSS, administration of IV thrombolysis, occlusion site, laterality, time of postprocedural CT, endovascular technique, contrast volume, or blood pressure measurements. In multivariable analysis, there was a trend toward a higher incidence of CS-NI with higher ASPECTS (8–10) and higher reperfusion (TICI 2b–3), but it did not achieve statistical significance. Otherwise, no independent association for CS-NI was found (Table 2).
DISCUSSION
In this retrospective analysis of 194 patients who underwent EVT for anterior circulation LVO stroke, we describe a phenomenon of CS-NI that consists of a benign and transient parenchymal enhancement on early NCCT after EVT, without evidence of infarct on follow-up imaging. CS-NI was identified in 13.4% (26/194); CS-I was observed in 26.8% (52/194); and both, in 5.6% (11/194) of patients who underwent EVT in our study. In contrast to CS-I, indicating permanent brain injury and poor outcomes, patients with CS-NI in our series showed a favorable clinical course with no post-EVT worsening.12,17⇓⇓-20
CS-NI is likely linked to a reversible, milder BBB disruption, differing from the more severe, potentially irreversible BBB disruption seen in CS-I. In addition to the BBB breakdown, the no-reflow phenomenon could contribute to contrast staining outside of the infarcted tissue. The no-reflow phenomenon describes a lack of blood flow at the capillary level despite established flow on the macrovascular level, preventing blood washout in the microcirculation and resulting in contrast retention and staining.21 The potential contribution of the glymphatic system to this phenomenon cannot be excluded.22 Existing evidence indicates that the functionality of the glymphatic system might be significantly compromised following AIS, resulting in reduced glymphatic perfusion and inadequate molecular clearance.23 We believe that CS is distinct from contrast-induced neurotoxicity/encephalopathy, which results from hyperosmolarity and direct neurotoxic effects caused by extravasated contrast and is marked by more pronounced and diffuse contrast enhancement, accompanied by abnormal MR imaging findings in the affected regions.24
No existing literature demonstrates the association between BBB integrity and the degree or type of contrast staining on conventional cross-sectional imaging. However, several studies, including perfusion imaging and electron microscopic examinations, have explored the regional heterogeneity of BBB disruption in AIS.25⇓-27 Krueger et al27⇓-29 found, through electron microscopic and immunofluorescence studies, that BBB injury extends to the penumbral areas, which showed less structural BBB damage than core infarct regions. Other studies revealed regional variations in BBB disruption between the core infarct and the penumbra on perfusion imaging, with the penumbra regions showing reduced BBB disruption.30
In the current study, CS-NI is characterized by mild parenchymal enhancement on noncontrast CT, with an average HU of 40, which was significantly lower than the CS-I (53 HU) and lower than what is typically described in the literature for contrast staining after EVT (mean, 63.4 [SD, 17.0] HU; range, 39–140 HU).9 Furthermore, CS-I is typically seen in the basal ganglia, whereas CS-NI was exclusively located in the cerebral convexities, which typically represent ischemic but not infarcted regions due to leptomeningeal collaterals (ie, penumbra).6,31 Parenchymal enhancement in CS-NI in our series was typically accompanied by increased attenuation in the CSF spaces, which could be related to the progressive clearance of the extravasated contrast molecules to the CSF spaces and/or the glymphatic system.23 Staining of the parenchyma and sulci in a given region can mimic cerebral edema and may be mistaken for irreversible tissue damage/infarction (Figs 2 and 3). CS-NI on CT may represent a phenomenon similar to that of the hyperintense acute reperfusion marker, which is a delayed enhancement of the subarachnoid or subpial space observed on postcontrast FLAIR MR imaging.32
Despite the statistical nonsignificance, the increased incidence of contrast staining in patients with kidney disease might also result from the direct effects of kidney disease on the integrity of the BBB or due to impaired renal function with a significantly prolonged half-life of the contrast medium (exceeding 16 hours compared with around 2 hours in patients with normal kidney function).33,34 Additionally, sustained exposure to microvascular risk factors, including kidney disease, hypertension, diabetes mellitus, amyloid angiopathy, and inflammatory conditions, may potentiate the effects of ischemia on the cerebral microvasculature and increase the risk of staining.6,21,35 Most interesting, no association was found between the occurrence of CS-NI and the use of IV thrombolysis, the duration of the procedure, or the volume of contrast injected, which have been described to potentially compromise the integrity of the BBB.6,36⇓-38
There are several limitations to our study. First, the retrospective and single-center study design introduces selection bias. Despite using the Firth regression adjustment, the small sample size may not have the statistical power to expose potential small effects. Additionally, preprocedural perfusion imaging was not obtained for most of the included patients, preventing us from assessing the perfusion status of brain regions that developed CS-NI. Moreover, we could not evaluate collateral circulation because we performed only single-phase CTA in our patients. Furthermore, we relied on restricted diffusion on MR imaging as a surrogate for irreversible infarction (core infarct) without considering the possibility of restricted diffusion reversibility after EVT. Finally, systematic recording of microcatheter contrast injection distal to the occluded side was not performed.
CONCLUSIONS
CS can be seen outside the infarcted tissue after EVT of AIS and is likely due to a reversible milder degree of BBB disruption. Although potentially benign, knowing its characteristics is important because it may mimic pathologic conditions such as infarcted tissue and cerebral edema. Further studies are required to comprehend the role of the BBB after ischemic stroke and its potential role as a target for neuroprotection after stroke therapy.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received November 5, 2023.
- Accepted after revision February 3, 2024.
- © 2024 by American Journal of Neuroradiology