Abstract
BACKGROUND AND PURPOSE: Although the application of cryoablation to metastatic spinal tumors has been attempted, spinal cryoablation has the unique complication of cryogenic spinal cord injury. This study aimed to elucidate the conditions for the development of cryogenic spinal cord injury.
MATERIALS AND METHODS: Fifteen canines were used in this study. A metal probe was inserted into the 13th thoracic vertebral body. Cryoablation was performed for 10 minutes by freezing the probe in liquid nitrogen. The control canine underwent probe insertion only. Spinal cord monitoring, epidural temperature measurement, motor function assessment, and pathologic examination of the spinal cord were performed.
RESULTS: During the 10 minutes of cryoablation, the epidural temperature decreased and reached the lowest epidural temperature (LET) at the end of cryoablation. The LETs (degrees celsius [°C]) of each canine were −37, −30, −27, −8, −3, −2, 0, 1, 4, 8, 16, 18, 20, and 25, respectively. As the epidural temperature decreased, waveform amplitudes also decreased. At the end of cryoablation (10 minutes after the start of cryoablation), abnormal waves were observed in 92.9% (13/14) of canines. With epidural rewarming, the amplitude of the waveforms tended to recover. After epidural rewarming (2 hours after the start of cryoablation), abnormal waves were observed in 28.6% (4/14) of canines. The LETs (°C) of the canines with abnormal waves after epidural rewarming were −37, −30, −27, and −8. None of the canines with normal waves after epidural rewarming had any motor impairment. In contrast, all canines with remaining abnormal waves after epidural rewarming had motor impairment. In the pathologic assessment, cryogenic changes were found in canines with LETs (°C) of −37 −30, −27, −8, 0, and 1.
CONCLUSIONS: This study showed that 10-minute spinal cryoablation with LETs (°C) of −37, −30, −27, −8, 0, and 1 caused cryogenic spinal cord injury. There was no evidence of cryogenic spinal cord injury in canines with LET of ≥4°C. The epidural temperature threshold for cryogenic spinal cord injury is between 1 and 4°C, suggesting that the epidural temperature should be maintained above at least 4°C to prevent cryogenic spinal cord injury.
ABBREVIATIONS:
- CMAP
- compound muscle action potential
- LET
- lowest epidural temperature
- SCEP
- spinal cord–evoked potential
Metastatic spinal tumors can significantly compromise a patient’s quality of life because they can cause severe pain, pathologic vertebral fractures, and spinal cord compression.1 Therefore, local control of metastatic spinal lesions is essential to maintain a patient’s quality of life, particularly in patients with oligometastatic disease, which has a better prognosis.
Currently, the standard treatments for metastatic spinal tumors are based on surgical and radiation therapy. Surgical treatment is an effective procedure that can remove or reduce metastatic spinal lesions and provide spinal stability with instrumentation.2 However, surgery is usually not performed in patients with extensive metastatic disease burden and patients with poor functional status because of surgical invasiveness and the risk of developing complications.3 Therefore, the current mainstay of treatment for metastatic spinal tumors is radiation therapy. In addition, the emerging technique of stereotactic body radiation therapy is reported to be effective even for radioresistant tumors.
Recently, cryoablation using extremely cold conditions to destroy tumor cells has been widely used in tumor treatment for various organs. Cryoablation is increasingly being performed in musculoskeletal metastases and has been reported to provide sustainable local control.4,5 The advantages of cryoablation include direct visualization of the ablation zone, decreased periprocedural pain, and the ability to use multiple probes in variable configurations to create tailored ablation zones.6 Moreover, the efficacy of cryoablation has been confirmed in radiation therapy–resistant tumors such as renal cancer.7 In clinical practice, cryoablation has been used for metastatic spinal tumors.8
However, cryoablation of the spine may result in cryogenic spinal cord injury, a serious complication occurring when cooling causes irreversible damage to the spinal cord.9 Moreover, the conditions for developing cryogenic spinal cord injury remain unclear, so there are limited indications for the use of cryoablation in metastatic spinal tumors. To establish the safety of spinal cryoablation, it is important to identify the conditions for developing cryogenic spinal cord injury. This study aimed to determine the threshold epidural temperature for developing cryogenic spinal cord injury using a canine spinal cryoablation model.
MATERIALS AND METHODS
Experiments were performed on 15 canines (female Beagles) between 9 and 10 kg and 80 and 90 cm in length. All canines were acclimatized under the same conditions at the Institute for Animal Experiments, where they were housed individually in metal cages and provided with food and water ad libitum.
Anesthetic Technique
After the administration of medetomidine (30 µg/kg) and midazolam (0.3 mg/kg) into the paravertebral muscles, a catheter was inserted into the radial vein for drug administration and fluid replacement. The canines were intubated and artificially ventilated with 50% nitrous oxide and 50% oxygen. They were placed in the prone position on an operating table under IV anesthesia with propofol (0.2 mg/kg/min). Muscle relaxants were not used. A cannula (2.0-mm diameter) was inserted into the right femoral artery for repeat blood sampling and continuous blood pressure monitoring. Body temperature was maintained between 36°C and 37°C using a heating pad as necessary and monitored with a rectal temperature probe. Arterial blood gases were measured at 90-minute intervals. The metabolic and respiratory acid-base balance was controlled with supplemental IV NaHCO3 to maintain a pH of approximately 7.40. PaO2 was maintained above 100 mm Hg, and PaCO2 was maintained between 30 and 45 mm Hg by adjusting the respiratory volume. Concentrations of nitrous oxide and oxygen were constant throughout the procedure. Painful stimulation did not cause any increase in blood pressure during steady-state anesthesia. Systemic blood pressure was maintained between 90 and 120 mm Hg for all procedures. In cases with a hypotensive trend, the infusion solution was increased or the propofol flow volume was slightly decreased.
Freezing Device
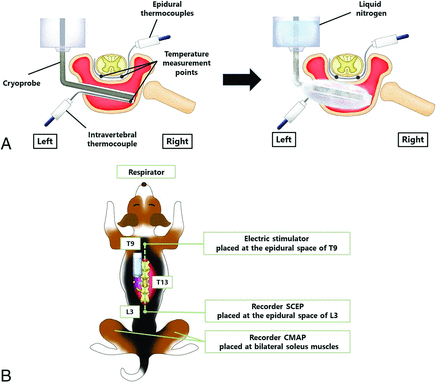
A freezing device was created for our experiment on the basis of a previous report.10 A 10-mL disposable syringe was incorporated into a 20-mL syringe to create a layer of air and prevent heat conduction from the syringe surface. A metal wire was placed at the tip of the syringe to form the cryoprobe. The metal wire tip was rapidly cooled via thermal conduction by injecting liquid nitrogen into the device (Fig 1A).
A, Details of cryoablation and thermal monitoring. B, Details of spinal cord monitoring.
Surgical and Cryoablation Procedures
After exposing the laminae from T11 to L2 through a posterior midline longitudinal incision, laminectomy at T13 and resection of the left proximal 13th rib were performed. After we exposed the left lateral aspect of the T13 vertebral body, a hole for the insertion of a cryoprobe was made using a 3-mm steel bar and a pedicle probe. A cryoprobe was inserted into the T13 vertebra, and 10-minute cryoablation was performed (Fig 1A). The intensity of cryoablation varied for each canine by changing the diameter and material of the metal probe (diameter range of probe: 1.2–2.0 mm; material of probe: copper or iron). An increase in probe size led to higher freezing intensity. Furthermore, changing the probe material from iron to copper amplified the freezing intensity due to differences in thermal conductivity (Online Supplemental Data). The control canine underwent probe insertion as a sham operation. In this procedure, 1 cycle of 10 minutes of cooling and passive thawing was performed with epidural temperature measurement and spinal cord monitoring performed until 120 minutes after the start of cryoablation. Ablated vertebrae were evaluated by a CT apparatus for small experimental animals (Model LaTheta LCT-200; Hitachi-Aloka) to confirm that the probe hole was in the appropriate position 7 days after the cryoablation procedure.
Thermal Monitoring
Two spatula-type thermocouples (MF-SP-K; AS ONE; range: −50 degrees celsius [°C] to 200°C) were inserted into the ventral epidural space of T13 on the same plane as the cryoprobe, measuring the temperature of the bilateral ventral epidural space. In addition, a sheath-type thermocouple (S1K05 × 300–2; TOHO ELECTRONICS; range: −50°C to 200°C) was attached to the tip of the cryoprobe to monitor the intravertebral temperature (Fig 1A). Each temperature was recorded every 10 seconds until 2 hours after the start of cryoablation and recorded using a data logger (GL240-SD; GRAPHTEC).
Compound Muscle Action Potentials and Spinal Cord–Evoked Potentials
Spinal cord function was evaluated using compound muscle action potentials (CMAPs)11,12 and spinal cord–evoked potentials (SCEPs)11,13 produced by spinal cord stimulation (Fig 1B).
CMAPs were recorded from the bilateral soleus muscles with each pair of needle electrodes (NE-110B; Nihon Kohden) using the tendon-belly method after spinal cord stimulation.14 The electrodes for stimulation consisted of 18-ga tubes with 2 fine platinum coil terminals covered with polyethylene insulation (USY-100-2PMC; Unique Medical). The stimulating electrode was placed on the posterior midline of the dura mater at the level of T9, introduced from the laminectomy site at T13. The stimulus condition had a rectangular waveform, a pulse duration of 0.5ms, an interstimulus interval of 2.0 ms, and a train of 5 pulses per stimulation at 1 Hz to evoke muscle action potentials. The stimulation intensity ranged from 2 to 3 mA and was set to 10% above the level that elicited the maximal amplitude. A band-pass filter was selected at 10–3000 Hz, and the mean summation was 10. A Neuropack ∑EMG-SYSTEM (MEB-5504; Nihon Kohden) was used to record the signals. CMAPs were recorded before cryoablation (control) and at 2.5, 5, 7.5, 10, 30, 60, and 120 minutes after the start of cryoablation. Amplitude was measured between the maximum positive and negative peaks. Decreases of >70% of the control CMAP amplitudes were considered abnormal.15 The CMAP reflects the activity of the corticospinal tract (mainly the lateral column) and the anterior horn cells of the spinal gray matter.
In the SCEP study, the stimulating electrode (USY-100-2PMC) was placed on the posterior midline of the dura mater at the level of T9, introduced from the laminectomy site at T13. A recording electrode (USY-100-2PMC) was placed on the posterior midline of the dura mater at the level of L3, introduced from the laminectomy site at T13. The spinal cord stimulation used a rectangular waveform for 0.2-ms duration at 5 Hz. Furthermore, stimulation intensity was set at a supramaximal strength. The band-pass filter was set at 10–3000 Hz, and the mean summation at 50. A Neuropack ∑EMG-SYSTEM (MEB-5504) was used to record the signals. SCEPs were recorded before cryoablation (control) and at 2.5, 5, 7.5, 10, 30, 60, and 120 minutes after the start of cryoablation. Amplitude was measured between the maximum negative and positive peaks of the first potential. Decreases of >50% in control SCEP amplitudes were considered abnormal.13,16 The SCEP mainly reflects the combined activity of the long tract in the dorsal and lateral columns.
Postprocedural Neurologic Examination
Postprocedural neurologic function was evaluated using a modified Tarlov scale17,18 at 1 day and 1 week after the cryoablation procedure. The scale used is presented in the Table.
Pathologic Examination
Seven days after the cryoablation procedure,18,19 the canines were euthanized using an IV potassium chloride bolus. The T13 vertebra, including the spinal cord, was removed and fixed in 10% formalin. The mean length of the T13 vertebral body (maximal anterior-posterior diameter of the vertebral body), width (maximal transverse diameter of the vertebral body), and height (central height of the vertebral body) were 7.8 (SD, 0.56) mm, 15.2 (SD, 0.69) mm, and 15.3 (SD, 0.85) mm, respectively. The specimens were demineralized with ethylenediaminetetraacetic acid and stained with H&E for pathologic evaluation. Observations were performed using a microscope (BZ-9000; KEYENCE).
RESULTS
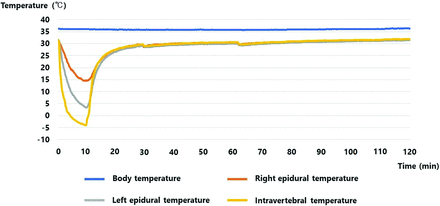
During the 10 minutes of cryoablation, the epidural temperature continued to decrease until the end of cryoablation. Overall, the left epidural measurement point had a lower temperature curve than the right. The left epidural temperature at the end of cryoablation (10 minutes after the start of cryoablation) was the lowest in each experiment. The lowest epidural temperatures (LETs) (°C) of each canine were −37, −30, −27, −8, −3, −2, 0, 1, 4, 8, 16, 18, 20, and 25. The epidural temperature increased after the end of cryoablation and almost recovered within 20 minutes after the end of cryoablation in all canines (Fig 2).
Temperature curves of the representative case with the lowest epidural temperature of 4 °C (case 6).
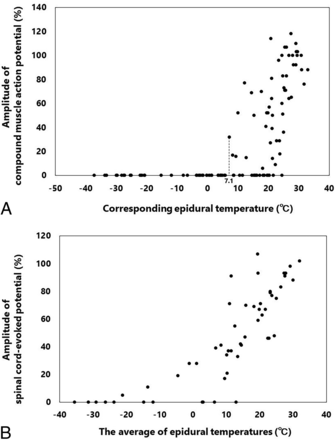
The relationship between the amplitude of spinal cord monitoring during cryoablation and the corresponding epidural temperature is illustrated in scatterplots (Fig 3). As the epidural temperature decreased, the waveform amplitudes of spinal cord monitoring also decreased. At the end of cryoablation, abnormal waves in CMAPs and SCEPs were observed in 85.7% (12/14) and 78.6% (11/14) of the canines, respectively.
A, Scatterplots illustrate the relationship between the left and right CMAPs at 2.5, 5, 7.5, and 10 minutes after the start of cryoablation and the corresponding epidural temperatures. B, Scatterplots illustrate the relationship between the SCEPs at 2.5, 5, 7.5, and 10 minutes after the start of cryoablation and an average of the corresponding left and right epidural temperatures.
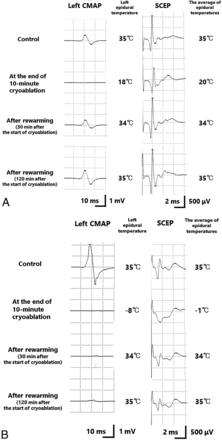
Thirteen canines had abnormal waveforms in either the CMAPs or SCEPs at the end of cryoablation; however, the amplitude of the waveforms recovered in 9 canines with the rewarming of the epidural temperature (Fig 4A). After epidural rewarming (2 hours after the start of cryoablation), abnormal waves of CMAPs and SCEPs were observed in 28.6% (4/14) and 21.4% (3/14) of the canines, respectively. In cases with LETs of −37°C, −30°C, and −27°C, abnormal waves of CMAPs and SCEPs remained after epidural rewarming. In the canine with an LET of −8°C, abnormal waves of CMAPs remained after epidural rewarming (Fig 4B).
Change of the CMAP and SCEP waveform in representative cases with the lowest epidural temperature of 18 °C (case 3) (A) and –8 °C (case 11) (B). A, The CMAP and SCEP waveforms decreased as the epidural temperature dropped. However, the waveforms recovered with rewarming of the epidural temperature. B, The CMAP and SCEP waveforms decreased as the epidural temperature dropped. The waveform of the CMAP did not completely recover after rewarming of the epidural temperature.
All canines with normal waves on spinal cord monitoring after epidural rewarming had no hindlimb motor impairment with grade V on the modified Tarlov scale on day 1 and week 1 after the procedure. In contrast, all canines that showed abnormal waves in spinal cord monitoring after epidural rewarming had grade III or lower hindlimb motor impairment on the modified Tarlov scale on day 1 and week 1 after the procedure.
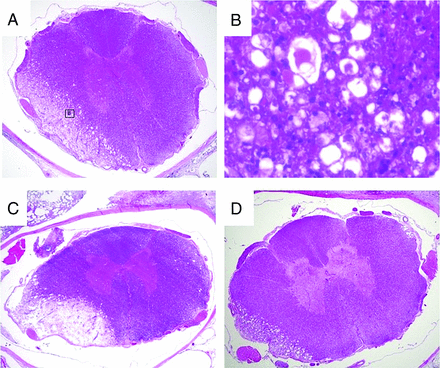
During the pathologic assessment of the spinal cord, cryogenic changes were detected in the canines with LETs (°C) of −37, −30, −27, −8, 0, and 1. In the lesion of cryogenic change, there were characteristic findings of acute spinal cord injury, including the proliferation of glial cells, neuronal vacuolation (spongiosis), and dilated myelin sheaths containing hypereosinophilic swollen axons (spheroids) (Fig 5).
The pathologic findings of the spinal cord in H&E staining. A, The pathologic specimen of the spinal cord in the representative case with the lowest epidural temperature of −8 °C (case 11) illustrates the cryogenic change in the left lateral and anterior columns (2× magnification). B, In lesions of cryogenic change, there is the proliferation of glial cells, neuronal vacuolation (spongiosis), and dilated myelin sheaths that contain hypereosinophilic swollen axons (spheroids) (60× magnification). C and D, The pathologic specimens of the spinal cord in cases 7 (C) and 8 (D). In these 2 cases with the lowest epidural temperatures of 1 °C (case 7) and 0 °C (case 8), the pathologic evaluation shows cryogenic spinal cord injuries, though spinal cord monitoring after epidural rewarming and the postprocedural motor function were normal. The specimens of the spinal cord in cases 7 (C) and 8 (D) illustrate the cryogenic changes in the left lateral and anterior columns (2× magnification).
A summary of the results, including intravertebral and epidural temperatures at the end of cryoablation, amplitude change of spinal cord monitoring, postprocedural modified Tarlov scale, and pathologic findings, are described in the Online Supplemental Data.
The sensitivity and specificity for detecting pathologic spinal cord injury in spinal cord monitoring combined with CMAPs and SCEPs during cryoablation were 100% (6/6) and 12.5% (1/8), respectively. After epidural rewarming, the sensitivity and specificity in spinal cord monitoring for detecting pathologic spinal cord injury were 66.7% (4/6) and 100% (8/8), respectively. The sensitivity and specificity of detecting postprocedural motor impairment in spinal cord monitoring after epidural rewarming were 100% (4/4) and 100% (10/10), respectively.
DISCUSSION
This study was the first experimental study to focus on elucidating the conditions under which cryogenic spinal cord injury develops. This study included conventional assessments, such as an observation of motor function and pathologic evaluation of the spinal cord, as well as an electrophysiologic assessment of the spinal cord. The effects of cryoablation on the spinal cord were investigated in detail by real-time monitoring of the spinal cord function.
To date, 2 experimental studies have been conducted on the safety of spinal cryoablation. Wallace et al20 examined the thermal protective capacity of the vertebral cortex and the accuracy of MR imaging for monitoring the ablation area using a sheep model. de Freitas et al19 performed cryoablation of porcine vertebrae and verified the feasibility of cryoablation of the spine. However, these 2 studies did not examine the conditions under which cryogenic spinal cord injury develops.
In the current study, findings of cryogenic spinal cord injury were observed in all cases with an LET of −8°C or lower. These results may be associated with extracellular ice formation. Water in the extracellular space crystallizes as the temperature drops to approximately −7°C.21 The extracellular fluid becomes hypertonic because of extracellular ice formation, causing cell dehydration and desiccation, which results in cell death. An experimental study using a rat model showed extensive axonal damage with loosening and edematous changes in interstitial tissue in the spinal cord after freezing at −8°C for 15 minutes,22 supporting our hypothesis that the withdrawal of water because of extracellular ice formation is associated with cryogenic spinal cord injury. Hence, spinal cryoablation with an LET of −8°C or lower, with extracellular ice crystal formation, may have a considerable risk of cryogenic spinal cord injury.
In all cases with an LET of −3°C or higher, intraprocedural spinal cord monitoring waveforms recovered after epidural rewarming, and the postprocedural motor function was normal. However, the pathologic evaluation revealed cryogenic spinal cord injury in 2 cases with LETs of 0°C and 1°C (Fig 5C, -D). In these 2 cases, cryogenic spinal cord injury was observed in a temperature range in which extracellular ice formation did not occur.
There is a similar phenomenon in the peripheral nerves called nonfreezing cold nerve injury, in which cryogenic nerve injury develops at a temperature above the freezing point. A rat model study demonstrated pathologic cryogenic nerve injury and an irreversible reduction in nerve blood flow after local cooling of the sciatic nerve for 3 hours at 1°C to 5°C, showing that ischemia plays a principal role in the nonfreezing cold nerve injury.23 Moreover, the association between cooling with near-freezing temperatures and ischemia has been reported in the spinal cord. A canine experimental model reported that spinal cord blood flow decreased to 50% of the normothermic values during the cooling of the posterior dura mater to 3°C.24 Considering these results, cooling to near-freezing temperatures may pose a risk of spinal cord injury including spinal cord ischemia, even if it does not reach the freezing range of extracellular fluid.
There were no cryogenic spinal cord injury findings in all cases with LETs of 4°C or higher. This finding was consistent with the results of a canine experimental model in which no cryogenic spinal cord injury was reported after selective spinal cord cooling at a dorsal column temperature of 5°C to 6°C for 100 minutes.25 These results show that maintaining the epidural temperature above at least 4°C prevents cryogenic spinal cord injury during cryoablation for metastatic spinal tumors.
To the best of our knowledge, this study is the first reported experiment to visualize the electrical activity of the spinal cord during cryoablation using spinal cord monitoring. The application of spinal cord monitoring for cryoablation of metastatic spinal tumors has been attempted to compensate for the disadvantage of conventional ice ball monitoring, namely that only frozen areas can be visualized.8 The findings of this study may contribute to establishing optimal protocols for spinal cord monitoring during cryoablation of metastatic spinal tumors.
The current study showed that the sensitivity for detecting cryogenic spinal cord injury in spinal cord monitoring combined with CMAPs and SCEPs during cryoablation was relatively high, 100% (6/6), and there were no false-negatives. Therefore, if the normal waveform of spinal cord monitoring is confirmed during cryoablation, the development of cryogenic spinal cord injury can be excluded, providing useful intraoperative information. However, the specificity for detecting cryogenic spinal cord injury in spinal cord monitoring during cryoablation was low at 12.5% (1/8), because spinal cord monitoring during cryoablation shows transient waveform reduction because of cooling. Hence, setting a lower threshold for abnormal waveforms should be considered when using spinal cord monitoring to reduce the rate of false-positives. In the present study, if even the slightest waveform of CMAP was observed during cryoablation, the ventral epidural temperature was confirmed to be at least 7.1°C, with a low risk of developing cryogenic spinal cord injury (Fig 3A). Therefore, during cryoablation, defining waveform disappearance as an abnormality in CMAPs may be practical.
In contrast, after epidural rewarming, spinal cord monitoring showed better diagnostic accuracy for detecting cryogenic spinal cord injury (sensitivity: 66.7% [4/6]; specificity: 100% [8/8]). However, 2 false-negative cases (cases 7 and 8) resulted in a slightly low sensitivity. In both false-negative cases, most areas of cryogenic injury were located in the anterolateral part of the spinal cord (Fig 5C, -D), possibly making detecting the cryogenic damage difficult in spinal cord monitoring combined with CMAPs (which mainly reflect electrical activity in the lateral column of the spinal cord) and SCEPs (which mainly reflect electrical activity in the lateral and posterior column of the spinal cord). Additionally, as shown in case 11 in which the waveform recovered after epidural rewarming in SCEPs but not CMAPs, SCEPs are considered to have a lower LET cutoff value for waveform recovery after epidural rewarming than CMAPs. This finding may be because CMAPs are generally the most sensitive method among the various spinal cord monitoring procedures.26 Another possible reason may be that SCEPs mainly reflect electrical activity in the lateral and posterior columns of the spinal cord, making it difficult to detect cryogenic damage in the anterior part of the spinal cord accurately, compared with CMAPs.
Notably, for detecting postprocedural motor impairment, the sensitivity and specificity in spinal cord monitoring after epidural rewarming were quite high at 100% (4/4) and 100% (10/10), respectively. Intraoperative prediction for postprocedural motor impairment is crucial because motor impairment can directly affect activities of daily living and quality of life.27 Using spinal cord monitoring would contribute to preventing postprocedural motor impairment, which is associated with great benefits in cryoablation for metastatic spinal tumors.
Although spinal cord monitoring is useful for detecting cryogenic spinal cord injury, it has the disadvantage of many false-positives at low epidural temperatures during cryoablation. Therefore, if a certain degree of epidural temperature reduction is expected, such as in cryoablation of spinal metastases surrounding the spinal cord, the combined use of epidural temperature measurement would be desirable. Previous studies have demonstrated the feasibility and safety of thermal ablation with real-time monitoring of the epidural temperature for treating spinal metastases adjacent to the spinal cord.28,29 A thermocouple in contact with the spinal cord provides continuous thermal monitoring of the surrounding spinal cord. Precise prediction of the development of spinal cord injury is possible by combining this real-time thermal monitoring with the findings on the conditions for the development of cryogenic spinal cord injury.
However, accurate placement of the thermocouple for measuring epidural temperature is challenging using a percutaneous procedure. Additionally, the preventive measure applied to avoid cryogenic spinal cord injury, such as carbon dioxide epidural injection, may interfere with the accuracy of the measurement of epidural temperature. A previous study recommended the placement of several thermocouples, because a single thermocouple may not be able to reflect the area of the largest temperature change.30 On the contrary, when cryoablation is combined with open decompression surgery for spinal metastases, thermocouples and insulation material for protecting the spinal cord can be directly placed in an appropriate epidural position because the spinal cord is exposed intraoperatively. We believe that this combined therapy can not only enhance local tumor control and functional prognosis but also improve the safety of the cryoablation procedure.
This study had several limitations. First, only 1 cycle of cooling and thawing was performed, and 2 cycles of cooling and thawing are commonly performed in clinical spinal cryoablation.8 We focused on 1 cycle of cooling and thawing in this study because in an experiment with 2 cycles, we cannot determine whether the first, second, or both cycles caused the cryogenic spinal cord injury, making it difficult to identify the accurate epidural temperature at which cryogenic spinal cord injury develops. A future study is required investigating the relationship between the cooling and thawing cycles and the conditions for the development of cryogenic spinal cord injury. Second, this study evaluated the LET but did not examine the cooling rate. The cooling rate is a factor that determines the degree of cryogenic tissue damage,31 and this should be assessed in future studies. Third, this study sample was not strictly a spinal cryoablation model. The intravertebral temperature must be below −40°C, when cryogenic tumor death occurs, to establish an accurate spinal cryoablation model.32 However, to prepare the cryoablation models with various ranges of epidural temperatures, it was necessary to include the models with intravertebral temperatures above −40°C, because creating a significant difference between the intravertebral and epidural temperatures was difficult due to the small size of the canine model in this study. Fourth, healthy vertebrae were used for this cryoablation model. The cooling distribution may be different between healthy vertebrae and tumor vertebrae due to mixed tissue with tumor and bone marrow, lytic destruction, and extraosseous extension into the canal. This limitation needs to be considered when applying the results of the current study to cases of spinal metastasis in clinical practice. Fifth, in this experiment, the lateral approach was used for probe placement because with the use of the transpedicular approach, there was a significant risk of probe deviation into the spinal canal from the canine’s thin pedicle. This experimental system was designed to focus on investigating the conditions in which the cryogenic spinal cord injury develops, and this experimental system does not directly reflect clinical spinal cryoablation in which the transpedicular approach is commonly performed.
Despite these limitations, the findings of this study on the conditions for the development of cryogenic spinal cord injury contribute to establishing suitable monitoring methods and appropriate preventive measures for cryogenic spinal cord injury.
CONCLUSIONS
This study showed that spinal cryoablation with a duration of 10 minutes and LETs (°C) of −37, −30, −27, −8, 0, and 1 caused cryogenic spinal cord injury. In contrast, there were no cryogenic spinal cord injury findings in the cases with LETs of 4°C or higher. Therefore, the epidural temperature threshold for cryogenic spinal cord injury was considered between 1°C and 4°C, suggesting that the epidural temperature should be maintained above 4°C to prevent cryogenic spinal cord injury during cryoablation for metastatic spinal tumors.
| Grade | |
|---|---|
| 0 | Complete paraplegia with no hind extremity function |
| I | Minor joint movements |
| II | Major joint movements |
| III | Animal can stand |
| IV | Animal can walk |
| V | Animal can climb a 20° inclined plane |
Modified Tarlov scale
Acknowledgments
We are grateful to Suga Chikazawa for her assistance with the pathologic examination. We would like to thank Yoshihiro Mozumi for technical assistance with the experiments. We are grateful to Kotoe Kobayashi for her assistance with creating the figures and to the members of the Institute for Animal Experiments for their important contributions to the experiments.
Footnotes
This research was supported by a Grant-in-Aid for Scientific Research, grant No. 22K09303.
This funder did not play any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received September 16, 2023.
- Accepted after revision December 18, 2023.
- © 2024 by American Journal of Neuroradiology