SUMMARY:
The neurobiological mechanisms underpinning psychiatric disorders such as treatment-resistant major depression, post-traumatic stress disorder, and substance use disorders, remain unknown. Psychedelic compounds, such as psilocybin, lysergic acid diethylamide, and N,N-dimethyltryptamine, have emerged as potential therapies for these disorders because of their hypothesized ability to induce neuroplastic effects and alter functional networks in the brain. Yet, the mechanisms underpinning the neurobiological treatment response remain obscure. Quantitative neuroimaging is uniquely positioned to provide insight into the neurobiological mechanisms of these emerging therapies and quantify the patient treatment response. This review aims to synthesize our current state-of-the-art understanding of the functional changes occurring in the brain following psilocybin, lysergic acid diethylamide, or N,N-dimethyltryptamine administration in human participants with fMRI and PET. We further aim to disseminate our understanding of psychedelic compounds as they relate to neuroimaging with the goal of improved diagnostics and treatment of neuropsychiatric illness.
ABBREVIATIONS:
- DMN
- default mode network
- DMT
- N,N-dimethyltryptamine
- FC
- functional connectivity
- LSD
- lysergic acid diethylamide
- rs-fMRI
- resting-state fMRI
Classical serotonergic psychedelics, such as psilocybin, lysergic acid diethylamide (LSD), mescaline, and N,N-dimethyltryptamine (DMT), are in the midst of a resurgent wave of interest within the field of neuropsychiatry. Despite the growing interest in these drugs as a treatment option for treatment-resistant major depressive disorder, post-traumatic stress disorder, and substance use disorders, our collective understanding of the neurobiology underpinning psychedelic therapy significantly trails the enthusiastic use and claimed neuroplastic effects touted by these therapies.1⇓-3 Insight into the underlying mechanisms of this unique drug class is crucial to advance the conversation around psychedelic therapy in the context of psychiatric illness. Neuroimaging, with techniques such as fMRI and PET, can provide researchers and clinicians alike novel insights into the psychedelic-driven neurobiological changes at the individual level and help lead to a more mechanistic understanding of the treatment effect of psychedelic therapy.
Potent serotoninergic hallucinogens have been used for millennia as an adjuvant during ritual and ceremonial practices and have emerged as a novel class of potential therapeutics for psychiatric illness.4⇓⇓-7 Psilocybin is a prodrug, which, following dephosphorylation in the liver to psilocin, demonstrates agonism of 5-HT2A receptors and partial agonism of 5-HT1A and 5-HT2C receptors.8 LSD, another psychedelic under active investigation for its therapeutic benefits, was first synthesized by Alfred Hoffman in 1938, and demonstrates 5-HT2A agonism as well as partial agonism of dopaminergic receptors.9 The endogenous psychedelic substance DMT, found in plants and the human brain, mainly acts as a 5-HT2A receptor agonist but shows promiscuity for other serotonergic receptors.10,11 Finally, mescaline, the active component of peyote, has 5-HT2A receptor agonism, with some activity at adrenergic receptors.6 In all instances, binding and agonism of the 5-HT2A receptor are thought to be responsible for the intense subjective sensations (eg, out-of-body experiences, altered consciousness, mindfulness), as well as functional changes in brain activity (eg, increased neuronal firing) and network reorganization that correspond to receptor-mediated neuroplastic regulation induced by such compounds.12,13 Though 5-HT2A agonism is regarded as the principal method of action for hallucinogenic effects in psychedelics, there are conflicting interpretations regarding how receptor polymorphism or co-agonism may influence the hallucinogenic state, and controversially, if this is necessary for positive neuroplastic and behavioral outcomes.14⇓-16 This lack of consensus has hindered researchers from confidently defining the mechanistic action behind psychedelics, delaying their widespread clinical adoption.17 To synthesize findings in the primary literature, many reviews include studies that rely on subjective effects of psilocybin more so than the potential functional connectivity (FC) changes induced in the brain upon administration; others have sought to include studies utilizing measurements, such as CBF, or place their focus on psychiatric populations only.18,19 In this review, we address the paradigms of resting-state fMRI (rs-fMRI), task-based fMRI, or PET in the context of psilocybin, LSD, or DMT administration within a nonpsychiatric human population to focus on the baseline effect of this exciting and emerging drug class on FC in the brain. Second, we address the drug-specific neurobiological changes mediated by serotonergic receptor activation that have been measured using multimodal PET-fMRI neuroimaging. Finally, this review will address its own limitations and those of other studies and suggest future models to bridge the knowledge gap between diagnosis and prognosis in the context of psychedelic therapy.
METHODS OF LITERATURE SELECTION
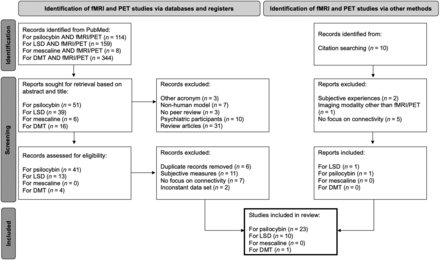
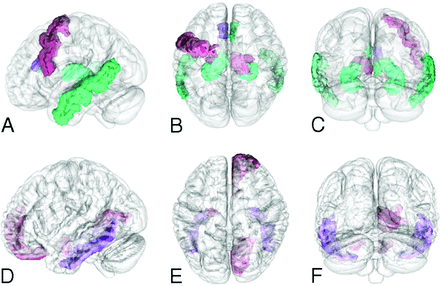
For this review, studies that reported the use of rs-fMRI and task-based fMRI were selected (Fig 1). To select fMRI articles, a PubMed search was conducted by using combinations of the keywords “psilocybin,” “LSD,” “DMT,” and “mescaline” in combination with “AND fMRI.” Though some clinical, psychedelic studies have used ayahuasca, only studies using DMT were considered to avoid muddled interpretation because of ayahuasca containing both DMT and harmala alkaloids, thereby acting as both serotonin agonist and monoamine oxidase inhibitor.20 A second PubMed search was conducted to select studies that used PET imaging as principal imaging technique by using the keywords “psilocybin,” “LSD,” “DMT,” and “mescaline,” also in combination with “AND PET.” No study that used mescaline fit the inclusion criteria for either fMRI or PET; thus, this review includes studies using LSD, psilocybin, and DMT, as represented in Figure 1.21 As the development of PET predates the development of fMRI imaging by more than 20 years, the articles included in this review regarding MR span from 2012–2023 and reflect the resurgence of psychedelic research that began in the early 2000s, whereas articles on PET span from 1997–2022 and are primarily based on studying models of psychosis.22 The imaging parameters of these studies are represented in the Online Supplemental Data, and the study design is reported in the Online Supplemental Data. This review only includes studies that strictly included nonpsychiatric human volunteers to establish a baseline understanding of how serotonergic psychedelics interact with functional brain connectivity (fMRI) and receptor biology (PET) for a higher spatiotemporal resolution.23 A visual representation of individual ROIs most affected by psilocybin and LSD is represented in Figure 2. Studies that used other methods of neuroimaging, such as CT, magnetoencephalography, single-photon emission CT, or electroencephalogram were excluded for a total of 34 articles.23
PRISMA 2020 flow diagram for method of selection of literature. Diagram of selection for current review, including searches of databases, registers, and other sources.
Visual representation of brain regions affected by psilocybin (A–C, sagittal, axial, and coronal, respectively) and LSD (D–F, sagittal, axial, and coronal, respectively). The connectivity, activation, or response of the visually represented regions is increased (green), decreased (red), or increased and decreased (purple), as figures in the selected literature. Regions affected by DMT are not represented because of insufficient ROI-based information. Figures produced by using a downloaded 4D functional human brain mask (https://nifti.nimh.nih.gov) and ROIs selected from DSI studio (https://dsi-studio.labsolver.org) human brain atlas.
DISCUSSION
Functional Neuroimaging Visualizes Psychedelic Alterations
FC can be measured by using fMRI, and together with blood oxygen level–dependent signal contrast, indirectly measure neuronal activity.24 This contrast captures signal related to functional activity in the corresponding brain regions activated during a task, referred to as task-based fMRI. In contrast, rs-fMRI does not employ any task-based activities and instead aims to characterize a baseline reading of brain network activity and synchrony, referred to as the default mode network (DMN). Thus, rs-fMRI allows analysis of the DMN, which is a particularly attractive technique when scanning patients with psychiatric conditions or those who are in a current psychedelic state and unable to correctly follow a task.24 The DMN has been proposed as a biomarker in fMRI studies for assessing behavioral outcomes based on initial (at-rest) brain coupling patterns.25,26 DMN FC alterations are featured prominently in psychedelic studies, and the clear effect of these compounds on behavior is likely to be associated with changes in the DMN. Thus, both task-based fMRI and rs-fMRI can establish the functional brain connectivity precedent of a patient by visualizing indirect spatiotemporal activity changes within and between brain networks. This enables researchers to monitor subsequent connectivity modifications, allowing for a deeper understanding of neurobiological consequences of these putative network connections following psychedelic ingestion.
Psilocybin.
Several studies have attributed the therapeutic potential of psilocybin to its ability to alter brain connectivity in limbic areas of the brain linked to emotional and memory response.27 This theory is supported by a 2015 study with 25 individuals, where functional imaging showed reduced activity, rather than an expected increase, in the right amygdala when presented with a negative or neutral visual cue to induce fear-based activation of the limbic system.28 A second study using the same 2015 data found that psilocybin reduced connectivity from the amygdala to the primary visual cortex, thereby decreasing the visual threat response.29 This decreased connection from amygdala to primary visual cortex was also observed in a follow-up study with 15 individuals, which found reduced connectivity from the frontal pole to the amygdala, and decreased connectivity between the amygdala and striatum upon a negative or neutral face visual cue.30 Together, these findings display the possibility of psilocybin treatment to reduce excessive amygdala reactivity, a symptom displayed in many psychiatric disorders.2,3 Finally, activation patterns were altered upon psilocybin administration in a study of 12 individuals finding reduced activation between the right amygdala and anterior cingulate cortex, a functional change that lasted up to 1 month postpsilocybin administration.31 Of interest, despite the noted reduction in activity particular to the amygdala, a 2014 rs-fMRI study of 25 individuals found increased signaling variation in the anterior cingulate cortex and hippocampus, pointing to the need for additional in-depth analyses of how psychedelics interact with connectivity and activation pattern changes that demonstrate region-specific changes throughout the brain.32
In addition to these effects, psilocybin has been shown to change intranetwork functional associations. The primary hallucinogenic experience following psilocybin administration is thought to be modulated by general decreases in neural activity.33 While a novel rs-fMRI analysis by Carhart-Harris et al33 of 15 individuals in 2012 found reduced global FC in the DMN, an analysis by Roseman et al34 of the same data set found increased connectivity between the visual and sensorimotor networks and resting-state (default mode) networks.33 The DMN, known for demonstrating increased activity in passive, reflective moments reveals a notably hyperactive FC in individuals with major depressive disorder.35,36 In addition to major depressive disorder, the DMN is also involved in social stress; psilocybin was shown to reduce feelings of social exclusion, not just by preventing them, but rather diminishing the strength of the negative experience. A 2016 rs-fMRI study by Preller et al37 demonstrated that this change was modulated by altered connections in the anterior cingulate cortex and medial frontal gyrus. Psilocybin induces an overall decrease in FC in the executive control network that correlates to, and, according to McCulloch et al (2022),24 predicts the positive personality changes lasting 3 months later. This decrease in executive control network connectivity is reproduced in a 2015 study that demonstrates a relationship between decreased amount of executive network nodes and increased subjective effects, measured as ego dissolution.38 Furthermore, the corresponding ego dissolution was found to be a result of psilocybin-induced decreased FC between the medial temporal lobe and other higher-level areas of the brain. Thus, the decreased connectivity seen in nonpsychiatric patients might validate a similar alteration that occurs in psychiatric patients. In a follow-up study using the 2012 data set from Carhart-Harris et al, psilocybin administration was associated with increased connectivity between the default mode and task positive networks.39 A 2020 rs-fMRI study by Mason et al40 also found increased connectivity between the DMN and salience or attention networks. Together, these reports demonstrate psilocybin can potentially alter connectivity between resting-state networks and active, task-based networks.
Madsen et al41 showed in a 2021 rs-fMRI study that the psilocin plasma level in blood negatively correlated with the level of network integration in both executive and DMNs, underscoring the role of acute effect of psilocin on the de-integration of networks. A 2020 study of 23 individuals showed 5-HT2A receptor agonism was associated with desynchrony of executive control and attention networks and an increase in connectivity at sensory regions, pointing to a delicate pattern of altered connections within and between the networks.42 These findings were independently confirmed during a third reanalysis of the 2012 Carhart-Harris et al study, even after controlling for the confounding potential of neuronal activity and cardiovascular overlap.32 Lastly, task-based fMRI demonstrated that psilocybin affects the claustrum; a 2020 study of 12 participants found decreased connections between the claustrum and DMN but an increased connection between the claustrum and task positive network.43 As the claustrum highly expresses 5-HT2A receptors and is involved in connectivity to the cerebral cortex, this finding is interesting but has not been replicated. Overall, fMRI studies demonstrate that psilocybin-induced region-specific increases in network connectivity between the DMN and the claustrum, sensorimotor, visual, and task-positive networks occur in conjunction with a global decrease in FC. In addition, psilocybin was found to induce elevated brain signaling at sensory regions, while concomitantly decreasing the brain’s ability to process associative input.33,34,44 In this case, brain signaling can be illustrated as dynamic measures of CBF and electrical activity using an fMRI-electroencephalogram or magnetoencephalogram and reflects the brain’s ability to reach flexible states of disorganization, as seen from psilocybin, causing altered states of “normal” consciousness.44 Together, this suggests that psilocybin results in an influx of sensory information along with an altered ability to effectively integrate these new inputs, thus creating intense perceptual effects.
LSD.
LSD has also been shown to induce unique alterations in resting-state and task-based fMRI. Unlike psilocybin, LSD induces altered sensory information flow in the thalamocortical pathway, by improperly filtering external and internal signals, creating an excessive influx of disintegrated information.45 This was validated in 2 rs-fMRI studies of 20 individuals where LSD administration induced hyperconnectivity between the precuneus and thalamus, and between primary sensory areas and thalamus, respectively.46,47 This latter finding was replicated in a 2018 rs-fMRI study of 24 individuals where increased connectivity was observed between the posterior cingulate cortex and thalamus, an outcome dependent on 5-HT2A receptor activation.48 A 2022 rs-fMRI study of 25 individuals demonstrated not only an increase in structural and FC between the right lingual gyrus and thalamus, but a decrease between the left auditory cortex, postcentral gyrus, and thalamus. Thus, rs-fMRI can highlight the unique, LSD-induced connectivity changes between the thalamus and lingual or proprioceptive areas of the brain.49 A generalized increase in hyperconnectivity is also demonstrated in a 2016 rs-fMRI study of 20 individuals where LSD induced increased connectivity between the primary visual cortex, prefrontal cortex and caudate.50 Another main area that seems to be affected by LSD administration is the frontoparietal cortex, which experiences increased FC as identified in 2 studies using reanalysis of previously published data.48,50⇓-52
DMT.
As with the LSD and psilocybin, the endogenous psychedelic DMT also demonstrates significant effects on human brain FC. Only 1 study was included in the present review to isolate the unique FC characteristics induced by DMT administration. A 2023 rs-fMRI study by Timmermann et al53 demonstrated a notable decrease in between-network segregation in frontoparietal, salience, and DMNs. However, an increase in global FC is seen throughout the brain, specifically in the frontoparietal, salience, and DMNs. This finding demonstrates the compound’s ability to cause paradoxical changes within and between functional networks.53 Of interest, this was a similar connectivity pattern observed upon psilocybin administration in an rs-fMRI study from 2020, where the DMN, attention, and salience networks also become altered.40 Though these 3 psychedelic drugs are used interchangeably in clinical treatment, the above fMRI studies suggest that each drug has unique effects on brain region and network connectivity, highlighting a need to more closely examine appropriate usages for each treatment.
Differences in Brain Activity and Affected Regions between the Psychedelics.
Functional imaging can isolate and characterize the unique effects of psilocybin and LSD on the brain. For example, despite their common agonist activity at 5-HT2A receptors, the right amygdala and claustrum appear to undergo significant changes in connectivity following psilocybin administration, whereas with LSD the most affected regions are the caudate, bilateral amygdala, and thalamus.28,47,48 Though a 2013 rs-fMRI study found an increase in thalamic connectivity, a 2022 rs-fMRI study of 18 individuals using voxelwise component analysis instead found that psilocybin caused a decrease in thalamocortical connectivity in the visual and DMNs.39,54 Nonetheless, this thalamic network hypoconnectivity differs from LSD’s effect, which shows increased connectivity throughout the thalamocortical pathway, implicating the cerebellum, insula, and lingual gyrus as well as sensory regions.46,48,55 The higher intensity of perceptual changes experienced following LSD administration in comparison with psilocybin coincides with this region-specific activation.
In addition to increased thalamic connectivity, LSD was shown in a 2017 study by Mueller et al56 to cause a significant decrease in activity of the left amygdala and right medial prefrontal cortex upon negative visual cues. Furthermore, a 2020 study by Bershad et al57 of 20 individuals showed LSD increased connectivity from the amygdala to the right angular gyrus, middle frontal gyrus, and cerebellum. This opposes multiple psilocybin studies that show decreased connections to and from the right amygdala.28,30,31,57 Perhaps unique to DMT, a decrease in integrity of global connections within networks associated with language is observed, while 2 studies using LSD find increased FC between language networks and other areas of the brain.51,53,55 Thus, DMT may possess distinct effects that should be addressed in future studies.50,51,58 While these 3 hallucinogens possess similar downstream effects, they all retain specific activity-dependent modifying properties. Though functional neuroimaging permits in vivo observation of the brain activity likely underpinning subjective hallucinogenic experiences, it does not provide a tailored measure of the psychedelic compounds’ serotonergic receptor and metabolic interactions, and thus prevents a more nuanced understanding of the neurobiology driving these changes.
PET Neuroimaging and Serotonergic Radiotracers
PET uses radioactive isotope tracers to measure metabolic changes and receptor binding alterations in the brain. PET thus serves as a molecular complement to the functional information derived from fMRI. To capture distinct, psychedelic-induced alterations at a neurometabolic and synaptic activity level in the context of imaging, the ROI needs to be considered alongside the selection of tracer. For example, psilocybin has been shown in murine models to increase dendritic spine attenuation and size in the frontal cortex; however, an open question is whether 5-HT2A activation is required to induce the observed increase in neural plasticity.13,14 Thus, the use of radioligands with specific agonism or antagonism for certain serotonergic receptors can help dissect the neurobiological mechanisms and sequelae associated with psilocybin or LSD administration.
11C Radioligands.
11C radioligands, such as 11C-MBL and 11C-Cimbi36, agonize the 5-HT2A receptor and can provide insight into psychedelic-driven receptor changes. Developed over the past 40 years, these ligands are particularly relevant for serotonergic psychedelic studies as they both show primary selectivity for 5-HT2A receptors in the cerebellum and cerebral cortex, 2 ROIs of these drugs.59,60 Indeed, 11C-MBL has been recommended over other carbon radioligands for serotonergic studies because of higher specificity for 5-HT2A receptors; treatment with 5-HT2A antagonist ketanserin blocks 11C-MBL binding potential except at the cerebellum. However, this recommendation was in the context of current uses for general serotonergic imaging and not specific to studies using psychedelics.61 Another interesting finding was that the radiotracer 11C-raclopride, a competitive D2/3 binding antagonist, displayed diminished binding potential following administration of psilocybin in the caudate and putamen in a 1999 study, suggesting that psilocybin may partially induce downstream release of endogenous dopamine release.62 Nonetheless, the binding strength of 11C can function as an indirect marker of in vivo serotonin levels through the level of 5-HT2A receptor occupancy. Furthermore, 11C-Cimbi36 showed greater sensitivity to 5-HT2A/2C receptor level changes but is associated with a low signal-to-noise ratio, which when coupled with the short half-life of 11C compounds, has somewhat limited its widespread adoption.63,64
A 2019 study of 8 individuals by Madsen et al65 using 11C-Cimbi36 PET to assess psilocybin occupancy of the 5-HT2A receptor was able to determine that high variability exists between each participant based on the dose–response curve. Higher occupancy of the neocortical serotonergic 5-HT2A receptor and higher levels of psilocin plasma concentration levels corresponded to persisting behavioral effects.65 This same data set was later reanalyzed in a 2022 study that looked at the binding capability of 11C-Cimbi36 before drug administration and found a direct relationship to mindfulness, a measure of behavioral change that lasted up to 3 months later.66 The lower binding capability in the right amygdala at baseline corresponded to higher levels of mindfulness 3 months after drug administration. Moreover, combining fMRI and PET can allow for a more complete image of individual baseline binding potential and subsequent functional outcomes; a 2022 study using both rs-fMRI and 11C-Cimbi36 PET was able to correlate neocortical 5-HT2A receptor binding at baseline with connectivity changes 3 months following psilocybin administration.24 Furthermore, a 2023 DMT study combined fMRI with a 5-HT2A receptor PET attenuation map and revealed a computationally verified relationship between 5-HT2A receptor signaling and subsequent FC outcomes following DMT administration. Though various 5-HT receptors were assessed by using different carbon radioligands, 11C-Cimbi36 was once again used to specifically focus on 5-HT2A receptor activity. Thus, the observed downstream effects of 5-HT2A receptor activation on within- and between-network connectivity in the brain emphasizes the role of combining fMRI and PET to gain a critical, personalized view of how a patient might potentially respond following the administration of serotonergic hallucinogens.53 In addition to 11C radioligands, 18F tracers have also been used in psilocybin studies to assess glucose uptake, synonymous with alterations in metabolic brain activity.
18F Radioligands.
18F-FDG-PET has emerged as a commonly used radiotracer to visualize general metabolic changes in resting-state networks because of its low reported incidence of signal interference secondary to neurovascular coupling and thus improved sensitivity and specificity.67 FDG is a glucose analog used as a quantitative measure of glucose utilization in the brain; therefore, 18F-FDG serves as an indirect measure of brain metabolic activity. A 1997 18F-FDG-PET study of 10 individuals found that psilocybin intake led to increased glucose uptake in fronto lateral and fronto medial regions, pointing to hypermetabolism in frontal regions of the brain.68 In contrast, a 1999 study of 32 individuals revealed that psilocybin induced a similar increase in glucose metabolism, but only at the right frontotemporal region of the brain, particularly at the anterior cingulate cortex. Furthermore, a decrease in glucose metabolism was noted in the right thalamus in the same study.69 Another PET ligand used to visualize serotonergic activity is the 5-HT2A antagonist radioligand 18F-altanserin. This tracer is pertinent to psychedelic imaging studies for its ability to assess 5HT2A receptor binding potential. Unlike 11C-Cimbi36, which has promiscuity for 5HT2C receptors, 18F-altanserin has a higher specificity for 5HT2A receptors.64 A 2009 study found that psilocybin caused an overall decrease in total distribution volume of 18F-altanserin, most drastic in the insula, frontal, and anterior cingulate cortex, demonstrating selective region-specific activity in regions implicated by psilocybin.60 Overall, PET radioligands enable sensitive and specific insights into neurometabolic and receptor-level activity changes in the brain, which optimizes the ability to study psychedelic mechanisms in a complementary manner to fMRI studies.
Limitations
A significant limitation of this review is its tailored nature; the selected published studies discussed herein only use fMRI or PET, and no other imaging technique, and only psilocybin, LSD, or DMT, and no other hallucinogens. Though many studies use similar data sets, the analyses performed are novel and provide additional insight into serotonergic psychedelic alternations in the brain. Further, the studies discussed had displayed an absence of demographic diversity, as most studies included only white individuals of European descent and limited sample sizes (all n ≤ 32 unless combining multiple data sets), especially the PET studies. While this review placed focus on nonpsychiatric human subjects, the reported literature also reports significant inconsistencies in studies between hallucinogen-naive and non-naive individuals. It remains to be seen if these differences reflect true differences in neurobiology or if hallucinogen naive and non-naive individuals experience significantly different subject experiences that drives the observed results. Many of the included studies also lean on subjective and metaphysical notions.18,19 The intrastudy interpretation of findings is thus confounded by an incomplete understanding of pharmacodynamics of serotonergic receptors, which highlights the need to study the long-term effects of psychedelic administration in the context of receptor internalization and (de)sensitization. Furthermore, the effect of receiving a sensationalized alternative treatment might increase the chances of conflated, positive experiences, but screening the participants carefully before inclusion seems to blunt this effect.70 The fMRI studies included varied significantly in methods of drug administration and methodologic analysis, which may lead to unwanted variation despite using identical data sets. While many of the studies included in this review were based on 3 initial studies, it remains important to consider replicating previous data sets by using independent data, as recommended by McCulloch et al.71 Another smaller but notable limitation includes the surprising number of studies not reporting handedness of participants. Overall, this focused review is a novel and necessary contribution illustrating the effect of serotonergic psychedelics in nonpsychiatric populations and emphasizes the importance of combining PET and fMRI to obtain a comprehensive baseline of psychedelic neuroimaging in the human brain.
CONCLUSIONS
This review covered the use of rs-fMRI, task-based fMRI, and PET imaging, which has allowed for a global, integrated understanding of the CNS as it relates to the functional changes occurring following serotonergic psychedelic therapy. The lacunas to accelerating mechanistic insight in the field of psychedelic biology can be addressed by employing a larger scale, with higher power, and by using multiparametric PET-fMRI technique for future psychedelic studies. This review suggests that combining PET and fMRI approaches will provide a comprehensive overview of the alterations seen in the psychedelic state and ultimately how these changes are associated with the observed treatment response. Ultimately, as the field begins to grow, it will be crucial to clarify and interpret the neurobiological effects of psychedelic therapies to increase insight into these specific mechanisms induced by psychedelics; this will hopefully complement current gaps of fMRI and result in a more personalized approach in the treatment of psychiatric illness.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
REFERENCES
- Received October 17, 2023.
- Accepted after revision November 20, 2023.
- © 2024 by American Journal of Neuroradiology