Abstract
BACKGROUND: Platelet function testing has been proposed to better adjust individualized antiplatelet treatment for patients undergoing endovascular treatment for intracranial aneurysms. Its clinical significance needs to be comprehensively evaluated.
PURPOSE: Our aim was to evaluate the impact of platelet function testing–guided versus standard antiplatelet treatment in patients receiving endovascular treatment for intracranial aneurysms.
DATA SOURCES: PubMed, EMBASE, and the Cochrane Library of clinical trials were searched from inception until March 2023.
STUDY SELECTION: Eleven studies comprising 6199 patients were included.
DATA ANALYSIS: ORs with 95% CIs were calculated using random effects models.
DATA SYNTHESIS: The platelet function testing–guided group was associated with a decreased rate of symptomatic thromboembolic events (OR = 0.57; 95% CI, 0.42–0.76; I2 = 26%). No significant difference was found in asymptomatic thromboembolic events (OR = 1.07; 95% CI, 0.39–2.94; I2 = 48%), hemorrhagic events (OR = 0.71; 95% CI, 0.42–1.19; I2 = 34%), intracranial hemorrhagic events (OR = 0.61; 95% CI, 0.03–10.79; I2 = 62%), morbidity (OR = 0.53; 95% CI, 0.05–5.72; I2 = 86%), and mortality (OR = 1.96; 95% CI, 0.64–5.97; I2 = 0%) between the 2 groups. Subgroup analysis suggested that platelet function testing–guided therapy may contribute to fewer symptomatic thromboembolic events in patients who received stent-assisted coiling (OR = 0.43; 95% CI, 0.18–1.02; I2 = 43%) or a combination of stent-assisted and flow-diverter stent placement (OR = 0.61; 95% CI, 0.36–1.02; I2 = 0%) or who changed from clopidogrel to other thienopyridines (OR = 0.64; 95% CI, 0.40–1.02; I2 = 18%), though the difference did not reach statistical significance.
LIMITATIONS: Heterogeneous endovascular treatment methods and adjusted antiplatelet regimens were limitations.
CONCLUSIONS: Platelet function testing–guided antiplatelet strategy significantly reduced the incidence of symptomatic thromboembolic events without any increase in the hemorrhagic events for patients undergoing endovascular treatment for intracranial aneurysms.
ABBREVIATIONS:
- EVT
- endovascular treatment
- HPR
- high on-treatment platelet reactivity
- IA
- intracranial aneurysm
- LTA
- light transmission aggregometry
- PFT
- platelet function testing
- RCT
- randomized controlled trial
In recent years, the use of endovascular treatment (EVT) for intracranial aneurysms (IAs) gained increasing attention due to its less invasive nature and associated improved clinical outcomes.1⇓⇓-4 Despite advancements in techniques and technology,1 thromboembolic events remain a major concern, leading to the adoption of conventional dual antiplatelet therapy as the standard regimen.5 However, a considerable proportion of patients (ranging from 15% to 55.3%) may develop high on-treatment platelet reactivity (HPR),6⇓⇓-9 which increases the risk of thromboembolic events.5,10
Platelet function testing (PFT) has been proposed as a means to adjust individualized antiplatelet strategies for patients with HPR.11 However, the use of PFT for EVT in patients with IAs remains controversial, and its clinical significance needs comprehensive evaluation. Several studies have demonstrated that PFT can reduce thromboembolic events in patients undergoing EVT for IAs.5,12,13 Conversely, other studies have not shown a significant reduction in such events14,15 and have suggested that the increased intensity of the antiplatelet treatment may lead to an increased incidence of bleeding events.5 Furthermore, there is inconsistency in the literature regarding the impact of PFT on clinical outcomes such as morbidity. Aoun et al16 found significantly reduced postoperative permanent morbidity, while Brinjikji et al17 found higher rates of morbidity in patients who underwent Pipeline Embolization Device (PED; Medtronic) replacement.
Unlike previous meta-analyses that focused on specific procedures (mainly PED treatment)18,19 or compared individual studies with or without PFT,19,20 our study uniquely selected studies reporting both PFT-guided and standard groups for any endovascular procedures. We aimed to comprehensively evaluate the effect of PFT on patients with IAs undergoing EVT by comparing those who underwent PFT and those who did not. This approach will fill a notable gap in the literature and add valuable insight to the existing body of research.
MATERIALS AND METHODS
Protocol and Registration
We followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guideline21 to perform this meta-analysis. This systematic review and meta-analysis was prospectively registered at the international prospective register of systematic reviews (PROSPERO) before searching.
Literature Research
PubMed, EMBASE, and the Cochrane Library of clinical trials data bases were searched from inception until March 2023 with no language restrictions. The terms were ([intracranial] OR [cerebral] AND [aneurysm]) AND ([platelet function test] OR [light transmittance aggregometry] OR [LTA] OR [vasodilator stimulated phosphoprotein] OR [VASP] OR [VerifyNow] OR [Thromboelastography]). The detailed search terms are available in the Online Supplemental Data.
Study Selection
Two independent reviewers (X.W. and L.L.) through EndNote (Version 9.3.2; https://endnote.com/downloads) performed manual screening and scanning for eligibility. The Population, Intervention, Comparison, and Outcomes Study (PICOS) principle was used to select included studies. The inclusion criteria were presented as follows: 1) Population: patients underwent endovascular treatment for intracranial aneurysm; 2) Intervention: patients received PFT and accordingly adjusted antiplatelet strategy; 3) Comparison: patients received standard antiplatelet strategy without taking the PFT; 4) Outcomes: symptomatic thromboembolic events, asymptomatic thromboembolic events, hemorrhagic events, intracranial hemorrhage, morbidity, and mortality; and 5) Study: cohort studies or randomized controlled trials (RCTs). We also hand-searched the reference lists of all included studies for potentially relevant citations. The outcome definitions of symptomatic thromboembolic events in the individual studies are listed in the Online Supplemental Data.
Quality Assessment
Two reviewers independently performed the quality assessment using the Newcastle-Ottawa Scale and the Cochrane Collaboration tool for assessing the risk of bias22 for cohort studies and RCTs, respectively. Disagreements on study quality assessment were discussed with another reviewer (Z.A. and Y.W.) until a consensus was reached.
Data Extraction
Two investigators (X.W. and L.L.) independently extracted data for eligible studies. The characteristic data obtained for each study included the first author, year of publication, study design, type of aneurysms, endovascular procedure, PFT method, initial and adjusted antiplatelet strategy, number of patients who received an adjustment in the adjusted group, sample size, and outcomes.
Statistical Analysis
ORs with 95% CIs were calculated with R statistical and computing software (Version 4.0.5; http://www.r-project.org). Heterogeneity across the studies was assessed using the Cochran Q test; the percentage of total variability attributable to heterogeneity was quantified by the I2 value. An I2 of <50% indicates low or moderate heterogeneity, and >50% indicates high heterogeneity.22 The random effects model with inverse-variance weighting was used for all analyses. P values < .05 were considered significant. Subgroup analysis was performed for symptomatic thromboembolic events and hemorrhagic events according to the endovascular procedure, initial antiplatelet treatment, adjusted strategy, and race. A difference between the estimates of these subgroups was considered significant for the P value between subgroups of <.10.23 To evaluate the stability of the results, we performed a sensitivity analysis by sequentially removing every single study from the pooled effect estimates including all cohort studies.
RESULTS
Three hundred seventy-nine studies were found after data base searching, of which 75 duplicated studies were excluded. After screening for title and abstract, 224 studies were removed and 80 were full-text screened (Online Supplemental Data). Eleven studies with 6199 patients in total were finally included in the systematic review and meta-analysis (Online Supplemental Data).5,12⇓⇓⇓⇓-17,24⇓⇓-27 One study5 was an RCT, and the other 10 were cohort studies. The endovascular treatment consisted of stent-assisted coiling, flow-diverter stent placement, and combinations of coiling, stent/balloon-assisted coiling, and flow-diverter stent placement. Types of platelet testing included VerifyNow (Accumetrics), light transmission aggregometry (LTA), multiple electrode aggregometry, INNOVANCE PFA-100 P2Y platelet function assay (Siemens), whole-blood aggregometry testing, and thromboelastography-platelet mapping. The proportion of patients who received antiplatelet strategy adjustment in the tested group ranged from 15.8% to 69.9%.
Quality Assessment
Only 1 study15 scored 4 on the Newcastle-Ottawa Scale because it was a conference abstract with little detailed information about the study design. The other 10 cohort studies scored 8–9 (Online Supplemental Data). For the RCT study, all potential biases were evaluated as low except for the performance bias for its open-label design (Online Supplemental Data).
Thromboembolic Events
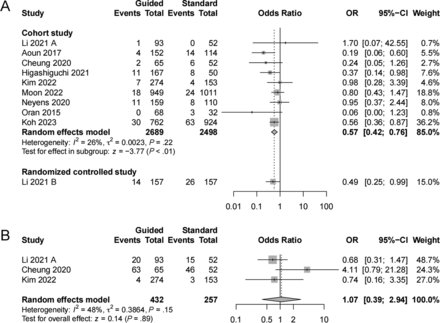
Ten studies5,12⇓⇓⇓-16,24⇓⇓-27 reported symptomatic thromboembolic events, including 9 cohort studies and 1 RCT. The guided group was associated with a decreased rate of symptomatic thromboembolic events compared with the standard group in cohort studies (OR = 0.57; 95% CI, 0.42–0.76; I2 = 26%) (Fig 1A). Three cohort studies13,14,25 reported asymptomatic thromboembolic events, and no significant difference was found between the 2 groups (OR = 1.07; 95% CI, 0.39–2.94; I2 = 48%) (Fig 1B).
Forest plot of symptomatic thromboembolic events and asymptomatic thromboembolic events comparing the PFT-guided group with the standard group. A, Symptomatic thromboembolic events. B, Asymptomatic thromboembolic events.
Hemorrhagic Events
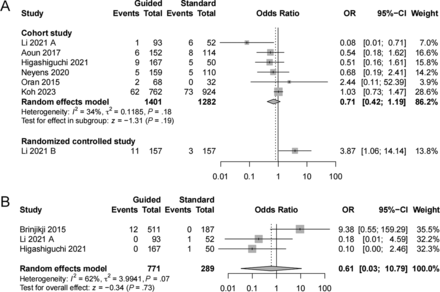
Seven studies5,12,16,24⇓⇓-27 evaluated hemorrhagic events, including 6 cohort studies and 1 RCT. We found no significant association between the guided and standard groups (OR = 0.71; 95% CI, 0.42–1.19; I2 = 34%) in cohort studies (Fig 2A). Similarly, no difference was found in intracranial hemorrhagic events (OR = 0.61; 95% CI, 0.03–10.79; I2 = 62%) (Fig 2B).
Forest plot of hemorrhagic events and intracranial hemorrhagic events comparing the PFT-guided group with the standard group. A, Hemorrhagic events. B, Intracranial hemorrhagic events.
Morbidity and Mortality
We found no association of morbidity (OR = 0.53; 95% CI, 0.05–5.72; I2 = 86%) between guided and standard groups (Online Supplemental Data). The mortality rate was comparable between the guided and the standard groups in cohort studies (OR = 1.96; 95% CI, 0.64–5.97; I2 = 0%) (Online Supplemental Data).
Subgroup Analysis (Data Base)
The results of the subgroup analysis for all cohort studies are presented in the Online Supplemental Data. Subgroup analyses were performed for symptomatic thromboembolic events and hemorrhagic events because other outcomes included only a few studies.
We found a slight but not significantly decreased risk of symptomatic thromboembolic events in the guided group compared with the standard group in subgroups using stent-assisted coiling (OR = 0.43; 95% CI, 0.18–1.02; I2 = 43%) and combined treatment (OR = 0.61; 95% CI, 0.36–1.02; I2 = 0%). There was no difference in symptomatic events between the 2 groups for patients who underwent PED stent placement (OR = 0.35; 95% CI, 0.03–4.64; I2 = 66%).
Additionally, the guided group showed a slightly lower incidence, though not significant, of symptomatic thromboembolic events compared with the standard group when clopidogrel was changed to other thienopyridines (OR = 0.64; 95% CI, 0.40–1.02; I2 = 18%). We found that neither changing the dual antiplatelet therapy dose (OR = 0.33; 95% CI, 0.05–2.12; I2 = 36%) nor adding other drugs into the antiplatelet strategy (OR = 0.35; 95% CI, 0.03–4.78; I2 = 64%) was associated with a lower incidence of symptomatic events.
PFT-guided treatment significantly reduced the incidence of symptomatic thromboembolic events in both Asian (OR = 0.62; 95% CI, 0.45–0.86; I2 = 0%) and white groups (OR = 0.32; 95% CI, 0.11–0.92; I2 = 5 5%).
Moreover, the PFT-guided group was not associated with a significant impact on hemorrhagic events regarding subgroups according to the endovascular procedure, adjustment strategy, or race compared with the standard group.
Sensitivity Analysis
The significance of the results changed when the study by Koh et al27 was excluded from the analysis of hemorrhagic events and the study by Brinjikji et al17 was excluded from the analysis of morbidity. The exclusion of any individual study included in the analysis did not significantly impact the overall findings of other outcomes (Online Supplemental Data).
DISCUSSION
The results of this meta-analysis, which included data from 6199 patients, indicated that the use of PFT-guided antiplatelet strategies in patients undergoing EVT for IAs significantly reduced the risk of symptomatic thromboembolic events without any increased risk of hemorrhagic events compared with the standard group. We found no significant impact of PFT-guided antiplatelet strategies on the incidence of morbidity and mortality.
In the subgroup analysis, we observed a notable trend toward a reduction in symptomatic thromboembolic events in the guided group of patients who received stent-assisted coiling or a combination of stent-assisted and flow-diverter stent placement, though the difference did not reach statistical significance. This finding suggests that PFT-guided therapy may have potential benefits for these specific subgroups of patients. However, the impact of PFT guidance on patients undergoing PED stent placement did not result in significant clinical outcomes, consistent with previous meta-analyses focused on PED-treated patients.19 Although a recent RCT5 reported reduced thromboembolic events with PFT guidance in this context (7.9% in the guided group and 20% in the standard group), further exploration and confirmation are needed due to the limited number of studies available for subgroup analysis and potential confounding factors.
Notably, we found that the replacement of clopidogrel with other adenosine diphosphate inhibitors had a tendency to reduce thromboembolic events, while other treatment adjustments did not show a significant association with this outcome. These findings highlight the importance of individualizing the antiplatelet strategy on the basis of PFT results, particularly in terms of selecting the most appropriate adenosine diphosphate inhibitor. Because it has been proved that high-dose clopidogrel could not overcome the clopidogrel hyporesponsiveness in a cardiac study,28 clinicians mainly replaced clopidogrel with other thienopyridines such as prasugrel12,15,29 and ticagrelor.5,13 Several studies suggested a better antithrombotic effect with a higher risk of hemorrhagic events of those novel P2Y12 inhibitors compared with clopidogrel.30⇓-32 The explanation for the elevated risk of hemorrhagic events could be the greater inhibition of platelet function and lower interindividual variability of the response to medication. Evidence from coronary trials revealed the association between those P2Y12 inhibitors and a higher hemorrhage incidence, so clopidogrel remained the first choice. On the other hand, patients with HPR who lack sufficient antiplatelet treatment might have an increased risk of in-stent thrombosis,33,34 resulting in additional operations. Hence, PFT provided an optimal way to monitor the platelet function and evaluate whether to switch to other antiplatelet strategies.
Furthermore, our analysis demonstrated the efficacy of PFT in reducing thromboembolic events without increasing bleeding risks in both Asian and white populations. This result suggests that PFT can be a valuable tool for optimizing antiplatelet therapy in diverse patient populations.
The phenotyping assay used varied among studies, highlighting the lack of consensus among different PFT tests in identifying patients with HPR. VerifyNow was the most commonly used PFT method among all the methods.35 It is a point-of-care PFT method that uses a concept similar to LTA, because LTA is known as the criterion standard for PFT but with limited clinical use due to its lack of standardization and time-consuming preparation process.35 At present, the ideal cutoff value for VerifyNow remains controversial and is mainly adapted or extrapolated from cardiology literature.35 There is no denying that the result of the PFT test itself is not stable due to many challenges, namely the transport of fresh blood for testing within a very short time frame.35 Future studies regarding more standardized methodologic-specific processes are needed to allow better reproducible tests and adequate thresholds.
Although the PFT has been proved useful for guiding antiplatelet escalation and de-escalation for antiplatelet strategy after percutaneous coronary intervention, it is recommended that it be performed only in specific clinical scenarios such as with a high thromboembolic/hemorrhagic risk instead of on a routine basis.36 To optimize the efficacy and safety of individualized antiplatelet treatment strategies, it is important to consider significant clinical and procedural variables when integrating PFT as an adjunct to antiplatelet therapy for patients underwent EVT treatment for IAs. Additionally, further evaluation is needed to focus on optimal PFT methods and establish appropriate cutoff values that accurately identify patients who would benefit from tailored antiplatelet treatment, appropriate adjustment strategies, and cost-benefit ratios in real-world implementation. Further well-designed controlled studies should be conducted to test this hypothesis.
To our knowledge, this study is the first systematic review and meta-analysis evaluating PFT in EVT for IAs for guiding antiplatelet treatment, incorporating studies that included both a PFT-guided group and a standard group. The confirmation of its beneficial effect in our study could allow further exploration of standardized PFT-guided antiplatelet strategies and their cost-effectiveness, which could have important implications for clinical practice.
However, it is important to acknowledge the limitations of our study. First, there were variations in procedural characteristics, such as the use of stent-assisted coiling or flow-diverter stents, as well as differences in antiplatelet strategies across the included studies. Despite these differences, the heterogeneity among studies for all outcomes was low-to-moderate. Furthermore, our subgroup analyses based on endovascular procedures, adjustment strategy, and race did not show significant differences between subgroups in terms of symptomatic thromboembolic and hemorrhagic events. Second, most of the included studies were cohort studies, with only 1 RCT available. Cohort studies using real-world data can provide valuable insight into the clinical relevance and overall benefit of PFT in guiding daily treatment decisions. These studies capture a broader patient population and reflect the effectiveness of PFT in routine clinical practice. Future well-designed RCTs focusing on different procedural or antiplatelet strategy settings would further contribute to the existing evidence base.
CONCLUSIONS
In patients undergoing EVT for IAs, the PFT-guided antiplatelet strategy significantly reduced the incidence of symptomatic thromboembolic events without any increase in the hemorrhagic events. Further studies focused on a standardized PFT-guided antiplatelet strategy and different procedures are needed.
Footnotes
Dr. Y. Wang and Z. An are the co-senior authors of this research.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received April 11, 2023.
- Accepted after revision June 1, 2023.
- © 2023 by American Journal of Neuroradiology