Abstract
SUMMARY: Previous studies have not found structural injury or brain malformations in infants and children with prenatal opioid exposure. As part of an ongoing study evaluating neuroimaging in infants with prenatal opioid exposure, we reviewed structural brain MR imaging in 20 term infants with prenatal opioid exposure and 20 term controls at 4–8 weeks of age. We found that 8 of the 20 opioid-exposed infants had punctate white matter lesions or white matter signal abnormality on structural MR imaging, and 2 of the opioid-exposed infants had a septopreoptic fusion anomaly. No controls had white matter injury or structural malformations. Our findings underscore the importance of clinical neurodevelopmental follow-up and the need for more comprehensive imaging and long-term outcomes research following prenatal opioid exposure.
Due to the ongoing opioid epidemic, >40,000 infants are now born exposed to opioids each year in the United States. The few previous studies evaluating structural neuroimaging in infants with prenatal opioid exposure have found no increase in the incidence of macrostructural injury or malformations.1,2 More recent studies have shown decreased head circumference,3 decreased brain volumes,4,5 and altered white matter microstructure6 in infants with prenatal opioid exposure compared with controls. Prior studies may have been confounded by coexposures because most opioid-exposed infants are also exposed to tobacco in utero,7 and a large proportion are also exposed to hepatitis C.8 Prenatal tobacco exposure is also associated with decreased head circumference9 and brain volumes.10⇓–12 There are no reports of neuroimaging after prenatal hepatitis C exposure in infants or children, but adults with active hepatitis C infection are known to have white matter changes.13 As part of a larger prospective study, we acquired structural brain MRIs in infants with prenatal opioid exposure and controls. Here we report our findings of white matter injury and congenital structural malformations in infants with prenatal opioid exposure, all of whom also had tobacco exposure and all of whom except one had hepatitis C exposure.
MATERIALS AND METHODS
As part of an ongoing prospective cohort study on functional brain connectivity in infants with opioid exposure, we acquired structural MR imaging in 4- to 8-week-old infants with confirmed prenatal opioid exposure and unexposed healthy term controls. Inclusion criteria for the opioid-exposed group included infants ≥37 weeks' gestation with known exposure to maternal buprenorphine or methadone during pregnancy and no known prenatal alcohol exposure. Inclusion criteria for controls were infants of ≥37 weeks' gestation with no opioid, alcohol, or illicit drug exposure during pregnancy confirmed by maternal urine toxicology screen and maternal history. Exclusion criteria for both groups included a 5-minute Apgar score of <7, any need for positive pressure ventilation at any time after birth, head trauma, and known chromosomal or congenital anomalies potentially affecting the central nervous system. Opioid-exposed infants were recruited from surrounding birth hospitals and the Cincinnati Children's opioid-exposed follow-up clinic. Controls were recruited from surrounding birth hospitals, flyers in surrounding pediatric offices, and e-mails sent to all hospital employees.
The study was approved by the Cincinnati Children's Hospital Medical Center Institutional Review Board, and written informed consent was obtained from parents/guardians. Images were acquired on a 3T Ingenia scanner (Philips Healthcare, Best, the Netherlands) with a 32-channel head coil during natural sleep using the feed-and-swaddle method. MR imaging included a sagittal 3D T1-weighted gradient-echo sequence (voxel size = 1 × 1 × 1 mm; scan time 3 minutes 6 seconds), an axial T2-weighted fast spin-echo sequence (voxel size = 1 ×1.11 × 1 mm; scan time 3 minutes 19 seconds), an axial 6-direction single-shot echo-planar DWI sequence (b-value = 800; voxel size = 2 × 2 × 2 mm; scan time = 1:47 minutes), and an axial 3D-SWI sequence (voxel size = 0.6 ×0.6 × 2 mm; scan time 4 minutes 3 seconds).
We reviewed electronic medical records from the infant's birth hospitalization for information including gestational age, birth weight/length/head circumference, sex, Apgar scores, results of maternal urine toxicology screen (done universally at the time of delivery at our surrounding birth hospitals), maternal medications and medical problems during pregnancy, maternal hepatitis C status, and length of hos-pital stay. Additional information reviewed for infants with opioid exposure included the results of neonate toxicology screen, the need for and type of treatment for neonatal abstinence syndrome, and the length of opioid treatment for neonatal abstinence syndrome, if necessary. At the time of the MR imaging visit, mothers filled out a questionnaire with self-reported information about their use of prescription medications, illicit drugs, alcohol, and tobacco during pregnancy.
MR images were reviewed by a single pediatric neuroradiologist blinded to clinical history. Initially a clinical read was reported to rule out structural injury, and then MRIs were scored in detail using a scoring system based on Kidokoro et al,14 which included white matter abnormalities (cystic white matter lesions, focal signal abnormality, corpus callosum thinning, dilated lateral ventricles), cortical gray matter abnormalities, and deep gray matter and cerebellar abnormalities. The MR imaging scores were based on all 4 sequences (T1, T2, DWI, and SWI).
RESULTS
We included 40 infants, 20 with prenatal opioid exposure and 20 controls. Eight of the 20 infants (40%) with prenatal opioid exposure had punctate foci of white matter injury or more diffuse white matter injury seen on the initial clinical read by the radiologist. Two of the opioid-exposed infants were also incidentally found to have septopreoptic fusion, a very mild form of holoprosencephaly. One of these 2 infants also had punctate white matter lesions. An example of the punctate white matter injury is shown in Fig 1. An example of the diffuse white matter injury is shown in Fig 2. No white matter injury or congenital structural malformations were observed in any of the control infants. One control infant had mildly enlarged extra-axial fluid spaces, one had a borderline small cerebellar vermis, and one had germinolytic cysts at the caudothalamic grooves bilaterally. One infant in each group (opioid-exposed and controls) had a small cerebellar germinal matrix hemorrhage. No other infants in either group had cortical gray matter, deep gray matter, or cerebellar abnormalities. Demographics and exposures for the 3 groups (prenatal opioid exposure with WM injury, prenatal opioid exposure without WM injury, and unexposed controls) are shown in the Table. As shown in the Table, all 8 infants with white matter injury also had tobacco exposure and 7 of the 8 also had hepatitis C exposure. No infant in any group was exposed to alcohol prenatally per parental report.
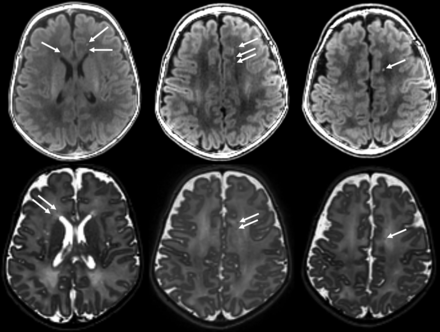
Example of punctate white matter injury seen in an infant with prenatal opioid exposure. Arrows show punctate white matter lesions. Upper row, T1-weighted images. Lower row, T2-weighted images.
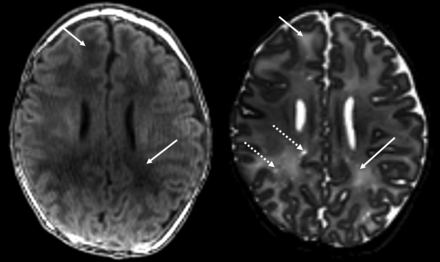
Example of diffuse white matter injury in infants with prenatal opioid exposure. Solid arrows denote abnormal T1 and T2 prolongation (which is bilateral but only denoted on the right). Dotted arrow demonstrates an example of perivascular space enlargement. Left, T1-weighted image. Right, T2-weighted image.
Characteristics of prenatal opioid-exposed and healthy term control study cohorts
DISCUSSION
White matter injury is described most commonly in infants born preterm, but it can also occur in full-term infants who sustain in utero insults during a susceptible period of white matter development.15 Both diffuse and punctate white matter lesions are thought to be related to the selective vulnerability of preoligodendrocytes.15 Punctate white matter lesions are small patches of increased signal intensity seen on T1-weighted imaging corresponding to areas of either small necroses, glial scars, or microhemorrhage.16 Punctate white matter lesions have also been reported in term and late-preterm infants with congenital heart disease, hypoxic-ischemic encephalopathy, and genetic disorders, and following neonatal surgery.17 In term infants with congenital heart disease, punctate white matter lesions are thought to be due to abnormal brain maturation due to disrupted blood flow and hypoxia from the cardiac lesion,18 leading to a delay in the maturation of preoligodendrocytes.19 Neurodevelopmental sequelae of punctate white matter lesions are variable, with some preterm infants with small isolated lesions (as in our cohort) reported as healthy, while others with more extensive lesion burden displaying motor and cognitive delays.20,21 Diffuse non-necrotic white matter injury is also commonly seen in preterm infants and also reflects disruption of the normal maturation of preoligodendrocytes.15,16,22 Outcomes after this diffuse mild injury are also variable. Studies that used qualitative diagnosis of diffuse signal abnormalities report normal developmental outcomes,23⇓–25 while studies that quantify it objectively or follow children with extensive hyperintensity report later cognitive and language delays.26,27
In our cohort of infants with prenatal opioid exposure, the etiology of the white matter injury is unclear. All 8 mothers were on medication-assisted therapy (in methadone treatment programs and 4 in buprenorphine treatment programs) throughout pregnancy, and 3 mothers had used street drugs early in pregnancy per maternal report and review of maternal urine toxicology. All of the mothers smoked during this pregnancy, and all except 1 of the mothers were positive for hepatitis C. WM injury has not been previously reported in the literature in association with prenatal opioid, tobacco, or hepatitis C exposure.
Two of the infants with prenatal opioid exposure were also incidentally found to have a septopreoptic fusion anomaly. This anomaly has been described in a single case series in the literature as the mildest form of holoprosencephaly, in which the septal and preoptic regions are fused and the rest of the brain undergoes normal cleavage.28 Cleavage of the prosencephalon normally occurs between days 18 and 28 of gestation.29 Holoprosencephaly has been associated with various genes and also environmental factors such as maternal diabetes, maternal alcohol use, and maternal infections.30 One of the 2 infants with this malformation had a mother with “borderline gestational diabetes” during her pregnancy, but the other had no record of diabetes in the maternal or infant chart. We could not find any literature describing an association between prenatal opioid exposure and holoprosencephaly in animal models. This finding in 2 of our 20 patients could very well be coincidental because there are no other reported cases of this association in either the human or animal literature.
The few studies evaluating structural brain MR imaging in infants with prenatal opioid exposure have concluded that there is no increase in macrostructural injury compared with controls.1,2 One of these studies used a 1.5T MR imaging scanner with thicker slices (4 mm), which could explain the lack of findings,2 but the other used a 3T scanner with 1-mm slices,1 similar to our protocol. Studies using more advanced MR imaging techniques have documented smaller brain volumes,4,5,31,32 altered white matter microstructure,6,33,34 and decreased cortical surface area and thickness5 in opioid-exposed infants and children compared with controls. None of these studies controlled for maternal smoking, and many included mothers with polysubstance use during pregnancy. However, animal studies have consistently shown the effects of prenatal methadone and buprenorphine on neurotransmitter biosynthesis,35,36 neurogenesis,37 and white matter development,38,39 providing evidence that opioids themselves likely affect brain development.
Overt brain injury has not been reported in infants exposed to tobacco during pregnancy. However, studies have shown an association between prenatal tobacco exposure and smaller brain volumes10 and decreased cortical thickness.10,12,40 There is no information in the literature about perinatal hepatitis C exposure and the developing brain, especially in the large majority of infants who are exposed to the virus but do not acquire the infection. However, hepatitis C is known to invade the central nervous system and lead to neurotoxicity, including altered white matter integrity in adults.13
CONCLUSIONS
We found that 8 of 20 infants with prenatal opioid exposure, all of whom also had coexposure to tobacco and all except 1 who had exposure to hepatitis C, had mild white matter injury seen on structural MR imaging at 4–8 weeks of age, and 2 of the 20 opioid-exposed infants had a septopreoptic fusion anomaly. Our study was limited by small sample size, and further studies must explore these associations in a much larger population.
Footnotes
Dr Merhar was supported by National Institutes of Health KL2 TR1426.
Disclosures: Stephanie L. Merhar—RELATED: Grant: National Institutes of Health, Comments: KL2 TR 1426*; UNRELATED: Employment: Children's Hospital Medical Center (Cincinnati). Brenda B. Poindexter—RELATED: Grant: National Institutes of Health, Comments: National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development UG1 HD027853-27S1 Clinical Research Investigator Supplement Award*; UNRELATED: Employment: Cincinnati Children's Hospital. *Money paid to institution.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received July 1, 2019.
- Accepted after revision September 3, 2019.
- © 2019 by American Journal of Neuroradiology