This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
Graphical Abstract
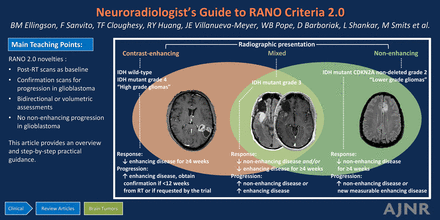
Abstract
SUMMARY: Radiographic assessment plays a crucial role in the management of patients with central nervous system (CNS) tumors, aiding in treatment planning and evaluation of therapeutic efficacy by quantifying response. Recently, an updated version of the Response Assessment in Neuro-Oncology (RANO) criteria (RANO 2.0) was developed to improve upon prior criteria and provide an updated, standardized framework for assessing treatment response in clinical trials for gliomas in adults. This article provides an overview of significant updates to the criteria including (1) the use of a unified set of criteria for high and low grade gliomas in adults; (2) the use of the post-radiotherapy MRI scan as the baseline for evaluation in newly diagnosed high-grade gliomas; (3) the option for the trial to mandate a confirmation scan to more reliably distinguish pseudoprogression from tumor progression; (4) the option of using volumetric tumor measurements; and (5) the removal of subjective non-enhancing tumor evaluations in predominantly enhancing gliomas (except for specific therapeutic modalities). Step-by-step pragmatic guidance is hereby provided for the neuroradiologist and imaging core lab involved in operationalization and technical execution of RANO 2.0 in clinical trials, including the display of representative cases and in-depth discussion of challenging scenarios.
ABBREVIATIONS:
- BTIP
- Brain Tumor Imaging Protocol
- CE
- Contrast-Enhancing
- CNS
- Central Nervous System
- CR
- Complete Response
- ECOG
- Eastern Cooperative Oncology Group
- HGG
- High-Grade Glioma
- IDH
- Isocitrate Dehydrogenase
- IRF
- Independent Radiologic Facility
- KPS
- Karnofsky Performance Status
- LGG
- Low-Grade Glioma
- MR
- Minor Response
- mRANO
- Modified RANO
- NANO
- Neurological Assessment in Neuro-Oncology
- ORR
- Objective Response Rate
- OS
- Overall Survival
- PD
- Progressive Disease
- PFS
- Progression-Free Survival
- PR
- Partial Response
- PsP
- Pseudoprogression
- RANO
- Response Assessment in Neuro-Oncology
- RECIST
- Response Evaluation Criteria In Solid Tumors
- RT
- Radiation Therapy
- SD
- Stable Disease
- Tx
- Treatment
- © 2024 by American Journal of Neuroradiology