Abstract
BACKGROUND AND PURPOSE: The association between infarct location and hemorrhagic transformation of acute ischemic stroke after mechanical thrombectomy is not understood. We aimed to evaluate the association between CTP-based ischemic core variables at admission and hemorrhagic transformation after a successful thrombectomy.
MATERIALS AND METHODS: We retrospectively analyzed patients who underwent endovascular thrombectomy for acute anterior circulation large-vessel occlusion between October 2019 and June 2021. We enrolled 146 patients with visible ischemic core on pretreatment CTP who had successful reperfusion. The ischemic core infarct territories were classified into the cortical and subcortical areas and then qualitatively and quantitatively analyzed by CTP. Logistic regression and receiver operating characteristic curve analyses were performed to determine the association between ischemic core variables and hemorrhagic transformation.
RESULTS: Of the 146 patients analyzed, 72 (49.3%) had hemorrhagic transformation and 23 (15.8%) had symptomatic intracerebral hemorrhage. Multivariate analysis showed that subcortical infarcts were independently associated with hemorrhagic transformation (OR, 8.06; 95% CI, 2.31–28.10; P = .001) and subcortical infarct volume was independently linked to symptomatic intracerebral hemorrhage (OR, 1.05; 95% CI, 1.01–1.09; P = .039). The receiver operating characteristic curve indicated that subcortical infarcts can predict hemorrhagic transformation accurately (area under the curve = 0.755; 95% CI, 0.68–0.82; P < .001) and subcortical infarct volume can predict symptomatic intracerebral hemorrhage (area under the curve = 0.694; 95% CI, 0.61–0.77; P = .002).
CONCLUSIONS: Subcortical infarcts seen on CTP at admission are associated with hemorrhagic transformation in patients after successful thrombectomy, and subcortical infarct volume may influence the risk of symptomatic intracerebral hemorrhage.
ABBREVIATIONS:
- HI
- hemorrhagic infarction
- HT
- hemorrhagic transformation
- ICC
- intraclass correlation coefficient
- IQR
- interquartile range
- PH
- parenchymal hematoma
- ROC
- receiver operating characteristic
- sICH
- symptomatic intracerebral hemorrhage
Mechanical thrombectomy is a standard approach used in patients with acute ischemic stroke caused by anterior circulation large-vessel occlusion.1 Hemorrhagic transformation (HT) and symptomatic intracerebral hemorrhage (sICH) after thrombectomy may decrease or eliminate the benefits of reperfusion, resulting in poor functional outcomes for patients with acute ischemic stroke.2⇓⇓-5 The main mechanism of HT after thrombectomy is thought to be acute reperfusion injuries to the disrupted BBB.6,7 Data from the Contact Aspiration versus Stent Retriever for Successful Revascularization (ASTER) randomized trial showed that there was no significant lesion growth following successful recanalization with mechanical thrombectomy.8 Additionally, previous research reported that pretreatment BBB leakage within infarcted brain tissue before reperfusion was associated with HT.7 However, the evidence for the association between ischemic infarct location and size at admission and hemorrhagic events after thrombectomy is inconclusive.9⇓⇓⇓-13 A common limitation of previous studies was that they used the ASPECTS for grading the ischemic core, which does not provide detailed information on the location and volume of the ischemic core.
Our study sought to investigate the association between the pretreatment infarct core location and HT in patients with acute ischemic stroke after a successful thrombectomy. We classified infarcts into those in the cortical and subcortical areas, and we used CTP to qualitatively and quantitatively analyze core infarcts at admission. We hypothesized that the location and size of an infarct at admission are associated with HT after successful thrombectomy.
MATERIALS AND METHODS
Population Selection
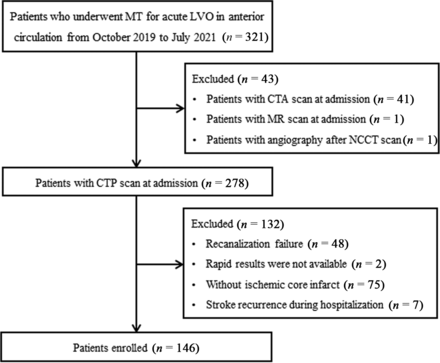
Between October 2019 and June 2021, we retrospectively reviewed consecutive patients who underwent mechanical thrombectomy for acute anterior circulation large-vessel occlusions using our stroke database. The selection criteria were as follows: 1) 18 years or age or older; 2) an initial NIHSS score of ≥6; 3) a prestroke mRS score of 0 or 1; 4) last-known-well time of <24 hours; 5) occlusion of the ICA and/or MCA M1 or M2; 6) successful recanalization after thrombectomy; and 7) ischemic core volume evaluated by CTP at admission (CBF < 30%) > 0 mL.14 The exclusion criteria were as follows: 1) occlusion of posterior circulation; 2) mechanical thrombectomy not performed or failed recanalization; 3) CTP not performed due to renal failure, contrast medium allergy, or other reasons; 4) nondiagnostic quality of CTP or ischemic core volume of 0 mL; and 5) stroke recurrence during hospitalization. Because our study is a retrospective study, the requirement for informed consent from patients was waived. The study was approved by our institutional review board. Figure 1 shows the flow chart for patient selection.
Patient-selection flow chart. MT indicates mechanical thrombectomy; LVO, large-vessel occlusion.
Clinical Evaluation
We collected and reviewed demographic and clinical information including age, sex, medical history (hypertension, diabetes mellitus, myocardial infarction, hyperlipidemia, atrial fibrillation, history of ischemic stroke, smoking, coagulation index, baseline NIHSS score, baseline ASPECTS, treatment with IV alteplase before thrombectomy, and blood pressure at admission), imaging data, procedural details, workflow, and clinical outcomes at 90 days. The NIHSS score was used to evaluate stroke severity. A CT scan was obtained within the first 24 hours after the procedure and repeated after 5–7 days to discriminate HT from contrast staining, which disappears on a follow-up CT.15 HT subtypes (HI 1, HI 2, PH 1, and PH 2) were classified according to the European Cooperative Acute Stroke Study II (ECASS II) criteria.16 sICH was defined as hemorrhage seen on CT that was accompanied by deterioration in a patient’s neurologic status and an increase in the NIHSS score (≥ 4 points).16 Clinical outcomes were assessed with the mRS score; a good outcome was defined as an mRS score of 0–2 at 90 days after treatment. The mRS scores at 3 months were obtained from the clinic records or through telephone interviews.
Image Analysis
Multimodal CT-based images, including noncontrast CT, CTP, and postprocessed series, are routinely obtained in patients with suspected acute ischemic stroke at our institution. The automated ASPECTS with a standardized 10-point scale was obtained from noncontrast CT. We processed perfusion images with the commercial software RAPID (iSchemaView) and automatically obtained the colored parametric CTP maps. The volumes of the ischemic core infarcts (CBF < 30%) were obtained from the CTP maps. All CTP images were visually inspected, and qualitative assessments of the core infarcts were performed. Ischemic core infarct patterns were classified as follows: 1) involving the subcortical area (regardless of whether the cortical area was involved); and 2) involving the cortical area only. The subcortical infarct areas include the caudate, lentiform nucleus, internal capsule, and insular ribbon. The quantitative assessment of the cortical and subcortical core infarcts was performed by manually segmenting parametric CTP maps using the open-source software ITK-SNAP (http://www.itksnap.org). The collateral status from DSA was determined using the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology guidelines. A simplified dichotomized judgment was used to improve interobserver agreement: good collaterals (grade 3–4) and poor collaterals (grades 0–2).17 The imaging data were assessed by 2 experienced neuroradiologists who were blinded to the clinical treatment and outcomes. A third neuroradiologist helped them reach a consensus if there were any discrepancies between the 2 neuroradiologists. The results of 3 representative cases are shown in the Online Supplemental Data.
Intra-Arterial Therapy
According to current guidelines, within 4.5 hours of stroke onset, patients are eligible for IV recombinant tPA before endovascular therapy. Endovascular thrombectomy was performed with the patient under local anesthesia or conscious sedation. A stent retriever was recommended as a first-line thrombectomy device, but other devices were also permitted. The modified TICI score was used to assess the recanalization grade. Successful reperfusion was defined as a modified TICI score of 2b or 3. Rescue therapies performed after failed mechanical thrombectomy included permanent stent placement, balloon angioplasty, and use of a glycoprotein IIb/IIIa antagonist. Patients who had successful recanalization received standard care in the stroke unit, and the systolic blood pressure was controlled (<180 mm Hg) for the first 24 hours after mechanical thrombectomy.
Statistical Analysis
Continuous variables were described as means (SD) or medians (interquartile range [IQR]), and categoric variables, as frequencies (percentage). The Shapiro-Wilk test and histograms were used to assess the normality of distribution. The Student t test and the Mann-Whitney U test were performed to analyze continuous data. The Fisher exact test was used for the analyses of categoric data. Significant clinical factors (P < .1) identified using univariate analyses were included in the multivariate logistic regression model to determine ORs and CIs. The receiver operating characteristic (ROC) curve analyses were applied to identify the effectiveness of significant variables for predicting HT and sICH after successful endovascular therapy. The Cohen κ was used to assess the interrater reproducibility for ischemic core location and collateral status evaluation. The intraclass correlation coefficient (ICC) was performed to evaluate the interreader agreement for the ischemic core volume segment. Results of the Cohen κ coefficient and the ICC were interpreted as follows: κ/ICC < 0.4 was considered poor reproducibility; 0.4 < κ/ICC ≤ 0.75 was considered fair-to-good; and 0.75 < κ/ICC ≤1.00 was considered excellent. The SPSS 26.0 (IBM) and MedCalc for Windows (Version 11.0; MedCalc Software) software packages were used for analysis. A P value < .05 was considered statistically significant.
RESULTS
Of the 321 patients with acute ischemic stroke reviewed during the study period, 146 patients met the inclusion criteria and were enrolled in the study. The mean age of patients was 71 (SD, 11.7) years, and 49.3% were women. The median baseline NIHSS score was 17 (IQR, 12–21), the median baseline ASPECTS was 7 (IQR, 5–8), and the median American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology grade was 2 (IQR, 1–3). The number of patients who had occlusions in the ICA, MCA, and ICA plus MCA was 47 (32.2%), 87 (59.6%), and 12 (8.2%), respectively. The causes of the stroke were cardioembolism (83, 56.8% of patients), large-artery atherosclerosis (38, 26.0% of patients), and undetermined (25, 17.1% of patients). Of the 146 enrolled patients who had a CTP at admission, 97 of them (66.4%) had symptom onset that was <6 hours. IV tPA was administered in 45 patients (30.8%) before mechanical thrombectomy.
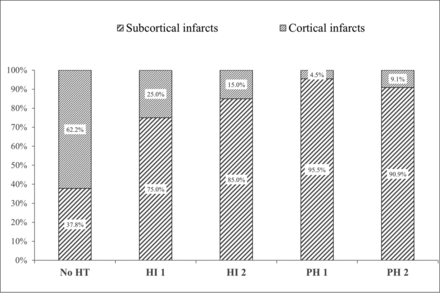
The assessment of infarct location and volume and collateral status showed excellent interrater agreement (κ = 0.908 and 0.826, respectively). The interreader reproducibility for the ischemic core volume segment was also excellent (ICC = 0.819). The CTP images of 92 patients (63.0%) showed a subcortical infarct. The median ischemic core volume determined by CTP maps was 19 mL (IQR, 9–44 mL), and the median infarct core volumes of the cortical and subcortical areas were 12 mL (IQR, 0–32 mL) and 7 mL (IQR, 0–14 mL), respectively. HT was identified in 72/146 patients (49.3%) after successful thrombectomy, and sICH was diagnosed in 23/146 patients (15.8%). In patients with HT, 5.5% (8/146) had HI 1, 13.7% (20/146) had HI 2, 15.1% (22/146) had PH 1, and 15.1% (22/146) had PH 2. The distribution of ischemic core infarct territories in patients with dichotomized HT and nonhemorrhagic transformation subtypes is shown in Fig 2. Patients with any HT subtype had a relatively high proportion of subcortical infarcts (75.0% in HI 1, 85.0% in HI 2, 95.5% in PH 1, and 90.9% in PH 2). This proportion was relatively low in patients with the nonhemorrhagic transformation subtype (37.8%). Functional independence at 3 months was achieved in 72 (49.3%) patients.
Distribution of ischemic core infarct patterns in patients with dichotomized HT and Non-HT subtypes.
The Online Supplemental Data show a comparison of characteristics between patients with and without hemorrhagic complications. Diabetes mellitus was more common in patients with HT than in those without HT (29.2% versus 10.8%, P < .01). Compared with patients without HT, patients with HT had a higher median NIHSS score (19 versus 16, P = .03), a lower median ASPECTS (6 versus 7, P < .01), worse collateral circulation (76.4% versus 36.5%, P < .01), and more passes of the retriever of ≥3 (34.7% versus 17.6%, P = .02). HT was more frequently observed in patients with subcortical infarcts (88.9% versus 37.8%, P < .01). Patients with HT had a larger median subcortical infarct volume compared with patients without HT (10 mL versus 0 mL, P < .01). In the multivariable regression analysis presented in the Online Supplemental Data, independent risk factors for HT were diabetes mellitus (OR, 4.54; 95% CI, 1.26–16.28; P = .020), core infarcts with subcortical involvement (OR, 8.06; 95% CI, 2.31–28.10; P = .001), poor collateral circulation (OR, 5.49; 95% CI, 2.15–14.01; P < .001), and >3 passes of a retriever (OR, 3.46; 95% CI 1.24–9.64; P = .018).
Patients with the sICH were older (76 versus 71 years of age, P = .04) and had a high prevalence of diabetes mellitus (39.1% versus 16.3%, P = .01), a higher baseline NIHSS score (21 versus 16, P < .01), a lower baseline ASPECTS (5 versus 7, P < .01), and worse collateral circulation (91.3% versus 49.6%, P < .01) than patients without sICH. Additionally, patients with sICH had more subcortical infarcts compared with those without sICH (87.0% versus 58.5%, P = .01). Subcortical infarct volume was higher in patients with sICH than in those without it (11 versus 6 mL, P < .01), whereas total core and cortical infarct volumes were not significantly associated with the occurrence of sICH (Online Supplemental Data). In the multivariable regression analysis, baseline ASPECTS (OR, 0.73; 95% CI, 0.57–0.93; P = .010), subcortical infarct volume (OR, 1.05; 95% CI, 1.01–1.09; P = .039), and collateral circulation (OR, 6.92; 95% CI, 1.47–32.73; P = .015) were significantly associated with sICH after successful thrombectomy (Online Supplemental Data).
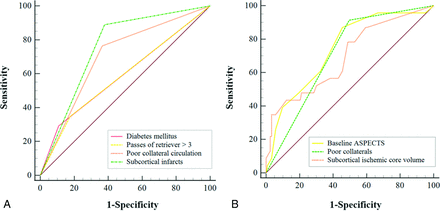
The ROC curves that were used for predicting HT and sICH are shown in Fig 3. The sensitivity and specificity for predicting HT and subcortical infarct involvement were 88.9% and 62.6% (area under the curve = 0.755; 95% CI, 0.68–0.82; P < .001), respectively. The cutoff subcortical infarct volume used for the prediction of sICH was ≥22 mL, and sensitivity and specificity were 34.8% and 96.8% (area under the curve = 0.694; 95% CI, 0.61–0.77; P = .002), respectively.
ROC analysis predicting HT (A) and sICH (B).
DISCUSSION
In this postthrombectomy patient cohort, we found that subcortical infarcts on admission CTP were associated with the occurrence of HT, and increased subcortical infarct core volume was associated with sICH. To the best of our knowledge, this study is the first to analyze the associations between CTP-based qualitative and quantitative core infarct variables and HT after successful thrombectomy.
The pathophysiologic mechanism of HT involves necrosis of cerebral infarct tissue, collateral circulation, BBB disruption, and infarct size.6,7,18⇓⇓⇓-22 Previous reports have shown an association between pretreatment BBB disruption and HT in patients with acute ischemic stroke after mechanical thrombectomy.7,22 However, the association of BBB disruption, infarction extent, and ischemic core at admission with HT after thrombectomy is unclear. Most studies reported that the ischemic core graded by ASPECTS is linked to HT or sICH after endovascular thrombectomy.11⇓-13 However, the multivariable regression analysis data from the Multicenter Randomized Clinical Trial of Endovascular Therapy for Acute Ischemic Stroke in the Netherlands (MR CLEAN) trial showed that the ASPECTS was not associated with HT or sICH.9 In this study, we quantified the pretreatment of ischemic core infarcts with CTP. We found that the total ischemic core and cortical infarct volumes did not show a positive association with HT or sICH after a successful thrombectomy. In contrast, subcortical infarct occurrence and volume were associated with HT and sICH risk, respectively. These findings may represent an imaging marker predictive of HT and sICH after mechanical thrombectomy that could help manage patients with acute ischemic stroke.
Reperfusion injury, including activation of the endothelium, excess production of oxygen-free radicals, inflammatory responses, leukocyte recruitment, increase in cytokine production, and edema formation could aggravate BBB disruption and potentially cause HT.23 Additionally, successful reperfusion after mechanical thrombectomy can cause acute hyperperfusion and hemodynamic changes to the infarct. The incidence of hyperperfusion after mechanical thrombectomy was relatively high in patients who achieved successful reperfusion.24 Postischemic hyperperfusion on arterial spin-labeled perfusion MR imaging is associated with HT in patients who underwent reperfusion therapy.25,26 These hemodynamic changes, especially in the lenticulostriate artery territories, may influence the rate of HT after reperfusion.27 Our findings support the hypothesis that subcortical infarcts, fed by perforating arteries, are associated with an increased incidence of HT.
Collateral circulation strongly influences the development of postrecanalization HT after acute large-vessel occlusion.9 The presence of unfavorable collaterals on angiography is one of the main reasons for HT after mechanical thrombectomy. Due to poor collateral flow, the hypoperfusion area may enhance the risk of HT after upstream recanalization. Arba et al7 found that pretreatment BBB disruption before reperfusion therapy was correlated with the extent of critically hypoperfused tissue. Subcortical tissue has poor leptomeningeal collaterals and more severe hypoperfusion compared with cortical tissue.18 BBB disruption and hemorrhagic conversion are more common in subcortical infarcts compared with cortical infarcts because of worse collateral anastomosis. Hence, our results show that subcortical infarction is associated with the occurrence of HT and increased subcortical core infarct volume is associated with sICH.
Several potential limitations should be mentioned in this study. First, it has the inherent limitations of its retrospective nature. Second, the boundaries between cortical and subcortical areas are sometimes difficult to distinguish accurately by manual infarct measurement. Artificial intelligence has enormous potential to achieve homogeneity in image interpretation in the future. Third, there may be certain differences between the core infarct volume obtained from CTP and the final true infarct size, though all patients underwent successful reperfusion. Fourth, the qualitative assessment by visual inspection could be feasible, but the quantitative infarct size assessment is not available immediately in clinical work.
CONCLUSIONS
This study shows that core infarct with subcortical involvement on CTP at admission is associated with an increased incidence of HT, and subcortical core infarct volume may influence the risk of sICH after a successful thrombectomy. The infarct location on CTP may be an imaging marker predictive of HT and sICH after mechanical thrombectomy that can help manage patients with acute stroke.
Footnotes
H. Ni and G.-D. Lu contributed equally to this work.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received September 15, 2022.
- Accepted after revision November 16, 2022.
- © 2023 by American Journal of Neuroradiology