Abstract
BACKGROUND AND PURPOSE: There are no validated imaging criteria for the diagnosis of progressive multifocal leukoencephalopathy in the cerebellum. Here we introduce the MR imaging shrimp sign, a cerebellar white matter lesion identifiable in patients with cerebellar progressive multifocal leukoencephalopathy, and we evaluate its sensitivity and specificity.
MATERIALS AND METHODS: We first identified patients with progressive multifocal leukoencephalopathy seen at Massachusetts General Hospital between 1998 and 2019 whose radiology reports included the term “cerebellum.” Drawing on a priori knowledge, 2 investigators developed preliminary diagnostic criteria for the shrimp sign. These criteria were revised and validated in 2 successive stages by 4 additional blinded investigators. After defining the MR imaging shrimp sign, we assessed its sensitivity, specificity, positive predictive value, and negative predictive value.
RESULTS: We identified 20 patients with cerebellar progressive multifocal leukoencephalopathy: 16 with definite progressive multifocal leukoencephalopathy (mean, 46.4 [SD, 9.2] years of age; 5 women), and 4 with possible progressive multifocal leukoencephalopathy (mean, 45.8 [SD, 8.5] years of age; 1 woman). We studied 40 disease controls (mean, 43.6 [SD, 21.0] years of age; 16 women) with conditions known to affect the cerebellar white matter. We defined the MR imaging shrimp sign as a T2- and FLAIR-hyperintense, T1-hypointense, discrete cerebellar white matter lesion abutting-but-sparing the dentate nucleus. MR imaging shrimp sign sensitivity was 0.85; specificity, 1; positive predictive value, 1; and negative predictive value, 0.93. The shrimp sign was also seen in fragile X–associated tremor ataxia syndrome, but radiographic and clinical features distinguished it from progressive multifocal leukoencephalopathy.
CONCLUSIONS: In the right clinical context, the MR imaging shrimp sign has excellent sensitivity and specificity for cerebellar progressive multifocal leukoencephalopathy, providing a new radiologic marker of the disease.
ABBREVIATIONS:
- JCV
- JC polyomavirus
- MS
- multiple sclerosis
- NPV
- negative predictive value
- PML
- progressive multifocal leukoencephalopathy
- PPV
- positive predictive value
- PRES
- posterior reversible encephalopathy syndrome
Progressive multifocal leukoencephalopathy (PML) is an opportunistic demyelinating disease in which the human JC polyomavirus (JCV) causes lytic infection of oligodendrocytes, astrocytes, and, rarely, neurons.1 It affects immunosuppressed patients with an impaired T-lymphocyte response, including patients with chronic lymphocytic leukemia, Hodgkin lymphoma, and HIV/AIDS.2⇓⇓-5 The increase in the PML incidence associated with immune therapies such as natalizumab for multiple sclerosis6⇓-8 underscores the importance of developing early, validated diagnostic criteria for PML.6⇓-8
Cerebellar and brainstem involvement in PML was identified 4 decades ago. There are few studies of the diagnostic specificity of pontocerebellar findings on MR imaging in PML.9,10 A punctate pattern of T2 and FLAIR hyperintensity is described in natalizumab-associated PML and in PML–immune reconstitution inflammatory syndrome.11,12 The hot cross bun sign, a cruciform T2 hyperintense signal in the midpons, has been noted late in the course of PML, affecting posterior fossa structures, when both the brainstem and the cerebellum have lost considerable volume (olivopontocerebellar atrophy). The hot cross bun sign was described initially in the cerebellar subtype of multiple system atrophy13,14 and is also seen in spinocerebellar ataxia, particularly spinocerebellar ataxia 1 and 2,15 and in variant Creutzfeldt-Jakob disease16 and cerebral vasculitis.17 Conditions other than PML affect the middle cerebellar peduncles, and disorders of immunocompromised patients such as toxoplasmosis, lymphoma, posterior reversible encephalopathy syndrome (PRES), neuro-Behçet disease, or HIV encephalitis may pose a diagnostic challenge. Cerebellar neuroimaging markers specific to PML may, therefore, aid diagnosis and preempt invasive procedures like brain biopsy.10
The shrimp sign was first proposed as a marker of cerebellar PML 25 years ago by N.V., who characterized it as a well-defined T2- or FLAIR-hyperintense and T1-hypointense lesion in the cerebellar white matter that abuts-but-spares the dentate nucleus and has the shape of a shrimp. It may also involve the hilum of the dentate nucleus. On axial MR imaging, the well-defined white matter lesion outlines the serrated, curvilinear-shaped dentate nucleus.
We designed this study to determine whether the shrimp sign is, indeed, a reliable indicator of cerebellar PML and to assess its sensitivity and specificity.
MATERIALS and METHODS
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the Partners Human Research institutional review board.
Study Population
Cohorts were drawn from medical record review of patients with T2-hyperintense cerebellar lesions at Massachusetts General Hospital between 1998 and 2019. Radiologic images acquired during clinical care were identified in Render, a searchable repository of radiology reports and images. Medical record review confirmed the diagnosis and captured demographic data.
Imaging Sequences
All MR imaging was performed as part of standard clinical care. Initially, axial planes of T1WI, T2WI, FLAIR, and, when available, postgadolinium T1WI were studied. After the preliminary stage of the study, we also examined T1WI in the sagittal and coronal planes.
PML Cohort.
Radiologic records were obtained using the search terms “progressive multifocal leukoencephalopathy” or “PML” and “cerebellar or cerebellum.” Using the American Academy of Neurology PML diagnostic criteria, we defined definite PML as patients with polymerase chain reaction positive for JCV from the CSF and pertinent clinical presentations and MR imaging findings.18 Possible PML was defined as patients who had not undergone lumbar puncture but were diagnosed using clinical presentations and MR imaging findings.
Control Cohort.
Patients with diseases known to affect cerebellar white matter were chosen as controls.19 Terms from broad categories (demyelinating lesion, leukodystrophy, cerebellar white matter + hyperintensity, middle cerebellar + white matter + hyperintensity) and specific categories (fragile X–associated tremor ataxia syndrome, PRES, cerebrotendinous xanthomatosis, Alexander disease, Langerhans cell histiocytosis) were searched twice, in combination with the terms “cerebellar” or “cerebellum.” Patients whose cerebellar pathology included hemorrhage, ischemic stroke, or space-occupying tumors (medulloblastoma, astrocytoma, ependymoma, glioblastoma) were excluded because these lesions are unlikely to be confused with PML. Patients with small, nonspecific cerebellar white matter lesions were also excluded. Because cases of MS and PRES were disproportionately higher than diagnoses in the rest of the group, we randomly chose 5 cases of MS and 4 of PRES and kept all other controls in the cohort.
Defining and Validating the Shrimp Sign
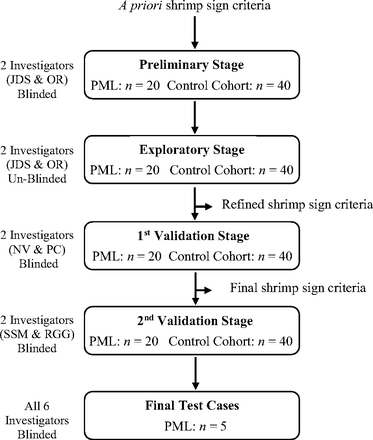
The shrimp sign was assessed through 4 stages (Fig 1). In the preliminary stage, O.R. (neuroradiology) and J.D.S. (neurology), blinded to the diagnosis, developed the shrimp sign criteria based on a priori knowledge and assessed its presence in all cases. The a priori inclusion criteria were the following: 1) presence of a well-defined lesion in the cerebellar white matter that is hyperintense on T2-weighted and FLAIR imaging and hypointense on T1-weighted imaging, and 2) the lesion abutting and sharply demarcating the dentate nucleus and outlining the dentate nucleus on axial MR imaging (Fig 2). The a priori exclusion criteria were the following: 1) invasion of the dentate nucleus by the white matter lesion, 2) a small and nonspecific lesion of the middle cerebellar peduncle, and 3) presence of hemorrhage, ischemic stroke, or space-occupying tumor in the cerebellar pathology. Permissible features included involvement of the white matter hilum of the dentate nucleus, lesions in the cerebral hemisphere and brainstem, and pontocerebellar atrophy. In PML present for a long duration (years), there may be prominent atrophy of the cerebellar hemispheres, dentate nuclei, middle cerebellar peduncle, and brainstem, sometimes with a hot cross bun sign in the pons.13,14 This olivopontocerebellar atrophy pattern may develop as a primary neurodegenerative disorder (neuronal PML) or as the late/burned-out stage of PML.
Flow diagram outlining the methodology for defining and validating the shrimp sign. In the preliminary stage, axial planes of T1-weighted imaging, T2-weighted imaging, FLAIR, and, when available, postgadolinium T1-weighted images were studied. In subsequent stages, these images were also examined in the sagittal and coronal planes.
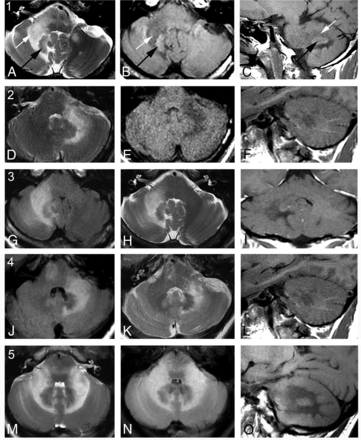
Representative MRIs from 5 patients with PML demonstrating the essential features of the MR imaging shrimp sign. Each row represents images taken from 1 patient, numbered cases 1–5: T2 axial (A), T1 axial (B), and T1 sagittal images (C). White arrows identify the white matter lesion; black arrows, the dentate nucleus. FLAIR axial (D), T1 axial (E), T1 sagittal (F), FLAIR axial (G), T2 axial (H), T1 coronal (I), FLAIR axial (J), T2 axial (K), T1 sagittal (L), T2 axial (M), FLAIR axial (N), and T1 sagittal (O) views. In case 5, the bilaterally symmetric shrimp sign is somewhat atypical in this patient with PML in the setting of confirmed HIV positive for CSF-JCV.
In the exploratory stage, diagnoses were revealed and the investigators reviewed the cases to refine the shrimp sign criteria. These revised criteria were used for the first validation stage, in which P.C. (neuroradiology) and N.V. (neurology) evaluated the MR images blinded to diagnoses. The investigators were aided by canonical images of positive and negative shrimp signs. Core inclusion and exclusion criteria were designated. The final criteria (Table 1) were subjected to a second validation stage by R.G.G. (neuroradiology) and S.S.M. (neurology), also blinded to the diagnoses. If reviewers disagreed, we erred on the side of specificity rather than sensitivity (ie, a determination of no for the presence of the shrimp sign prevailed over a yes determination). Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the shrimp sign criteria were assessed following the preliminary and validation stages. In these stages, 20 patients with PML and 40 controls were evaluated for the presence of the shrimp sign by each investigator.
Diagnostic criteria for the MR imaging shrimp sign of cerebellar PML
After the study had begun, 5 additional cases of PML were referred to Massachusetts General Hospital: 1 possible and 4 definite. These 5 were used as a test of the finalized shrimp sign criteria. All 6 senior investigators assessed the presence of the shrimp sign in these 5 cases, blinded to the diagnoses.
RESULTS
Patients with PML
The search for cases of PML generated 138 medical records. Of these, cerebellar white matter lesions were identified in 20 patients: 16 with definite PML (mean, 46.4 [SD, 9.2] years of age), 4 with possible PML (mean, 45.8 [SD, 8.5] years of age). See Table 2 for patient demographics. Of 16 cases of definite PML, 13 (81.25%) had HIV infection.
Patient demographicsa
Control Patients
Medical records of 716 controls were generated. We excluded 645 (90%) due to hemorrhage, ischemic stroke, tumors, duplicate records, and/or no sizeable cerebellar lesions. Of the remaining 71 controls, 25 had MS and 15 had PRES. The disproportionately greater frequency of these 2 diagnoses was addressed by randomly choosing 5 cases of MS and 4 of PRES. The final cohort included 40 control patients (mean, 43.64 [SD, 21.0] years of age). See Table 2 for demographics and Table 3 for diagnoses.
Diagnoses in the 40 patients in the control cohort
Development of Preliminary Diagnostic Criteria
Assessment of a priori criteria of the first evaluators (J.D.S., O.R.) resulted in 4 false-negative and 8 false-positive cases, a sensitivity of 0.8, specificity of 0.8, NPV of 0.89, and PPV of 0.67 (Table 4). After unblinded re-examination of the false-negatives, we noted the importance of evaluating all 3 cardinal planes. We determined that the white matter lesion outlining the dentate nucleus must encompass ≥50% of the nucleus for a positive diagnosis. If there are multiple white matter lesions, they must abut the nucleus, but they do not have to be contiguous. Review of false-positive cases prompted us to add cavitation within the white matter lesion as an exclusion criterion, along with T1 isointensity of lesions and overt atrophy of the dentate nucleus. Bilateral lesions were found to be a permissible atypical feature in PML. An important consideration in the differential diagnosis is HIV encephalopathy without PML. In these cases, the cerebellar white matter lesions are hazy and ill-defined; whereas they may be hyperintense on T2WI or FLAIR imaging, they are T1 isointense (not hypointense). Furthermore, the lesions are diffusely distributed or bilaterally symmetric in the cerebellar and cerebral white matter, further distinguishing them from PML.
Sensitivity and specificity of the MR imaging shrimp sign of cerebellar PMLa
Validation Stage 1
Assessment of the revised shrimp sign criteria resulted in 4 false-negatives and 1 false-positive, a sensitivity of 0.8, specificity of 0.98, PPV of 0.94, and NPV of 0.91 (Table 4). Imaging features of fragile X–associated tremor ataxia syndrome may resemble cases of PML with bilateral shrimp signs. We modified our criteria to highlight the permissible atypical feature of bilateral, usually asymmetric white matter lesions but draw attention to fragile X–associated tremor ataxia syndrome as a possible PML imaging mimic. Following review, we also amended the criteria, recognizing that when all core inclusion and exclusion criteria are met, minimal mass effect and enhancement following contrast administration remain compatible with the PML shrimp sign.
Validation Stage 2
By means of the amended criteria, the shrimp sign was evident in 17 of 20 patients with PML (85%). Of the 3 false-negatives, 1 had possible PML and 2 had definite PML. There were no false-positives; the shrimp sign was absent in all 40 controls. Assessment of the validity of the final shrimp sign criteria (Table 1) thus resulted in a sensitivity of 0.85, specificity of 1, NPV of 0.93, and PPV of 1 (Table 4). In the 17 patients with PML with the shrimp sign, it was noted at the first clinical presentation in 15 (88.2%), in all 3 patients with possible PML, and in 12/14 with definite PML. In the remaining 2 patients, the shrimp sign developed within a year of the PML diagnosis.
Final Test Cases
In the 5 additional PML patients evaluated using the final criteria there was unanimous agreement. Of the 4 definite PML cases the shrimp sign was present in 1 and absent in 3 (2 without cerebellar involvement, and 1 with end-stage olivopontocerebellar atrophy, white matter attenuation, and marked nuclear atrophy). There was no shrimp sign in the 5th patient with possible PML, who had leukoencephalopathy and patchy cerebellar white matter hyperintensities but was negative for JCV in the CSF.
DISCUSSION
The diagnosis of PML relies on clinical and MR imaging findings and detection of the JCV in the CSF by polymerase chain reaction. Because PML lesions may involve multiple brain areas, the clinical and imaging manifestations may vary.18 Characteristic features include multifocal distribution of patchy or confluent areas of white matter signal abnormality in the cerebral hemispheres, brainstem, and cerebellum. These lesions are T1 hypointense and T2 hyperintense4,20⇓-22 and typically do not enhance or cause mass effect proportionate to the volume of the lesion, though faint contrast enhancement and minimal mass effect are noted.21,22 Diffusion restriction may detect active lesions.22⇓-24
The absence of an MR imaging biomarker for cerebellar PML prompted us to assess whether the MR imaging shrimp sign may fill this gap. Results showed high sensitivity and NPV and excellent specificity and PPV, indicating that it is, indeed, a reliable imaging biomarker of cerebellar PML. The MR imaging shrimp sign is a distinct T2- or FLAIR-hyperintense and T1-hypointense lesion in the shape of a shrimp, located in the cerebellar white matter abutting-but-sparing the dentate nucleus. It outlines the serrated, curvilinear shape of the dentate nucleus, which stands out against this abnormal background.
Postmortem studies of cerebellar PML provide the neuropathologic basis for our imaging observations.9,25 One case revealed a very widespread demyelinating process involving mainly the right cerebellar hemisphere but also most of the pons and left cerebellum, with the typical morphologic characters of PML.9 Another demonstrated severe, confluent demyelination in the cerebellar white matter but, remarkably, sparing the dentate nucleus. Imaging in that case showed bilateral asymmetric lesions that were T2-hyperintense and T1-hypointense, consistent with what we now identify as the MR imaging shrimp sign.25 Histopathologic examination confirmed preservation of the dentate nuclei. The cerebellar white matter lesions were notable for complete loss of myelin sheaths, severe damage in the axons, infiltration of macrophages with increased microglial cells, and scarcity in oligodendrocytes and perivascular lymphocytes. These observations demonstrate the magnitude of cerebellar injury in a patient with PML and may explain the T1-hypointensity characteristic of the shrimp sign.
In a study of PML and relapsing-remitting MS, MR imaging findings and clinical features were analyzed to distinguish these disorders.26 Crescent-shaped white matter lesions were reported in 23% of the cerebellar PML cohort. Because that study examined only these 2 conditions, the specificity of this sign could not be assessed.
The finding of JCV in the CSF has been considered more sensitive and specific for PML than clinical and MR imaging findings. However, false-negative CSF JCV values are encountered when the immune system is relatively intact, as in MS and systemic lupus erythematosus.27,28 Patients with AIDS with PML on suppressive antiretroviral therapy may also have reduced or undetectable CSF JCV levels.29⇓-31 The good sensitivity and high specificity of the shrimp sign make it promising for the diagnosis and longitudinal study of cerebellar PML, complementing and supporting the clinical features. We note that disease survival in PML has increased with improved diagnostic techniques, earlier recognition, and the introduction of antiretroviral drugs.2,32 The shrimp sign was present at the onset of clinical disease in 75% (15/20) of our cohort of patients with PML, who were known retrospectively to have developed a cerebellar lesion at any point in their disease course, suggesting that the shrimp sign may be helpful for early diagnosis.
Limitations
By means of the final criteria, 3 cases in the original cohort of 20 patients who had known cerebellar PML were not diagnosed with the shrimp sign. One case did not have T1 hypointensity. This was a bona fide false-negative. Absence of the shrimp sign does not exclude a diagnosis of PML because the cerebellum may be spared or the radiologic findings may be only minimal. The remaining cases did not pass the criteria due to symmetric lesions across the cerebellar hemispheres. The reviewers were divided on these 2 cases: The neurologist used the shrimp sign because the symmetry was incomplete, but the radiologist was concerned about the symmetry. Because patients with widespread leukoencephalopathies, such as neuro-Behçet disease, Krabbe disease, or cerebrotendinous xanthomatosis may have more aggressive cerebellar involvement that extends to the middle cerebellar peduncles, brainstem, and white matter of the cerebellar folia, adhering to the diagnostic criteria will minimize false-positives, though it may result in occasional false-negatives. Consideration of the clinical details should help lead to the correct diagnosis.
A case in point is fragile X–associated tremor ataxia syndrome, which showed a striking resemblance to the shrimp sign. Unlike most of our cases of PML, fragile X–associated tremor ataxia syndrome cerebellar and middle cerebellar peduncle lesions were bilateral and symmetric. This X-linked disorder of older men with a premutation expansion (55–200) of the CGG repeat is characterized by slow evolution over a period of years of tremor, ataxia, peripheral neuropathy, erectile dysfunction, and cognitive decline, and the patient may have a grandson/grandnephew with the full mutation fragile X syndrome and daughters who are fragile X carriers.33 Imaging features notwithstanding, when the clinical context is considered, there should be little room for confusion between this genetic disorder and PML.
In most of our patients, PML occurred against a background of HIV. Of the 4 cases negative for HIV, the 1 case of possible PML and 2 of 3 cases of definite PML had the shrimp sign. PML is increasingly recognized in patients treated with immune therapies such as natalizumab and rituximab, but our study included only 1 patient with rituximab-associated PML, and this case was positive for the shrimp sign. Future studies will need to address the relative prevalence of the shrimp sign in patients with HIV-associated PML versus those with PML in the setting of other causes of immune compromise.
Severe dentate nucleus atrophy is an exclusion criterion for the shrimp sign, though it often accompanies olivopontocerebellar atrophy that may be observed in late-stage cerebellar PML. Dentate nucleus atrophy is generally not present in early cerebellar PML when the shrimp sign is evident. Measurement of the dentate nucleus diameter on clinical scans is technically challenging, and this issue precluded us from defining clear limits and quantitative comparisons of the nucleus diameters among cases. The subjective term “severe atrophy” in the exclusion criteria recognizes this reality and indicates that nucleus dimensions should be largely preserved relative to the remainder of the cerebellum.
Finally, we note that patients with PML may present with ataxia and cerebellar atrophy but no white matter lesions, reflecting JCV infection of the cerebellar granular cells, a constellation termed JCV granule cell neuronopathy.34 We caution that the absence of the shrimp sign in a newly ataxic patient with HIV does not preclude the possibility of JCV infection, even though the imaging is not indicative of PML.
Implications.
Our study is the first to identify a neuroimaging marker that is both sensitive and specific for cerebellar PML early in the disease. The clinical significance of our findings is highlighted by a recent case report of a patient with PML initially misdiagnosed with cerebellar ischemic stroke.35 Imaging at presentation revealed a focal lesion in the middle cerebellar peduncle sparing the dentate nucleus. During the next few months as the patient worsened, increasing lesion size prompted consideration of PML. By means of our shrimp sign criteria, earlier detection of PML may have been facilitated. Future studies can assess the reliability of our shrimp sign criteria in other cohorts and the potential for this sign to facilitate earlier detection and treatment of PML.
CONCLUSIONS
In this retrospective review of brain MR imaging studies in 2 cohorts, patients with known PML and those with other disorders associated with T2-hyperintense cerebellar lesions, the shrimp sign showed high sensitivity and NPV and excellent specificity and PPV for cerebellar PML. The shrimp sign is a valid and reliable radiologic biomarker for cerebellar PML and may aid in the diagnosis of cerebellar PML.
ACKNOWLEDGMENT
The assistance of Jason MacMore is gratefully acknowledged.
Footnotes
Noor Adra and Anna E. Goodheart are joint first authors.
This work was supported, in part, by the National Ataxia Foundation, the MINDlink Foundation, and Mrs Mary Jo Reston.
Disclosures: Anna E. Goodheart—UNRELATED: Consultancy: Harvard T.H. Chan School of Public Health, Comments: Medical consultant for an epidemiological study; Grants/Grants Pending: American Academy of Neurology training grant, Other: Massachusetts Alzheimer's Disease Research Center, Comments: Grant to support coursework and educational activities. Shibani S. Mukerji—UNRELATED: Grants/Grants Pending: National Institute of Mental Health, Harvard University Center for AIDS Research, Comments: K23MH115812, P30AI060354*; Jeremy D. Schmahmann—UNRELATED: Consultancy: Biogen, IQVIA; Patents (Planned, Pending, or Issued): author of the Brief Ataxia Rating Scale, Cerebellar Cognitive Affective Syndrome Scale, Patient-Reported Outcome Measures for Ataxia, all copyrighted to the General Hospital Corporation; Royalties: Oxford Academic, Springer, Mac Keith Press; Payment for Development of Educational Presentations: Continuing Medical Education lectures at Harvard Medical School; Other: site Principal Investigator on Biohaven Pharmaceutical clinical trials NCT03701399 and NCT03952806. *Money paid to the institution.
References
- Received June 24, 2019.
- Accepted after revision January 7, 2021.
- © 2021 by American Journal of Neuroradiology