Abstract
SUMMARY: Calcified pseudoneoplasms of the neuraxis are extremely rare non-neoplastic lesions that can exist anywhere in the CNS. Although benign, the lesions can cause substantial neurologic symptoms, typically related to mass effect on adjacent structures. Calcified pseudoneoplasms of the neuraxis can also mimic other entities such as calcified oligodendrogliomas and meningiomas. Nevertheless, the lesions can usually be strongly suggested at the time of imaging due to a number of fairly unique imaging characteristics. Here, the clinical presentation of a patient with a posterior fossa calcified pseudoneoplasm of the neuraxis is described, along with its imaging and pathologic features.
ABBREVIATION:
- CAPNON
- calcified pseudoneoplasm of the neuraxis
A 58-year-old man presented with a long-standing history of headaches. As a child, his symptoms were attributed to temporomandibular joint dysfunction; migraines were common during adulthood. Twenty-five years before presentation, he underwent CT of his head after a traumatic injury, which revealed a posterior fossa abnormality described as being “scar tissue,” per the patient (Fig 1). An MR imaging performed approximately 2 decades later showed a highly calcified lesion that appeared stable compared with the prior CT. The patient ultimately presented to our institution with progressively worsening headaches that were not successfully managed with over-the-counter medications.
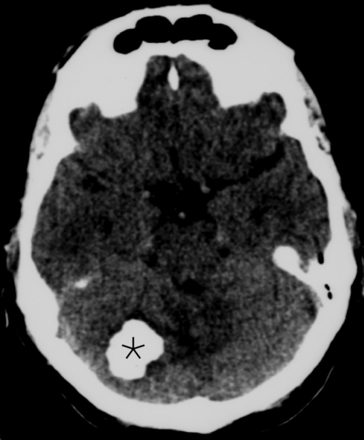
Initial CT scan of the mass obtained 15 years before presentation at our institution. The images demonstrate a densely calcified mass in the right posterior fossa (asterisk), without substantial local mass effect.
Imaging
Imaging revealed a densely calcified extra-axial mass in the posterior fossa, which appeared to originate from the tentorium. The mass had mildly increased in size since the initial CT. MR imaging demonstrated markedly hypointense intralesional signal on T2 and FLAIR. Modest internal enhancement was observed, which was superimposed on heterogeneous intrinsic T1 hyperintensity. More substantial enhancement was also observed around the margins of the lesion. Mild associated mass effect and vasogenic edema were present, with partial effacement of the fourth ventricle. There was also a suggestion of a dural tail, leading to the favored diagnosis of calcified meningioma (Fig 2).
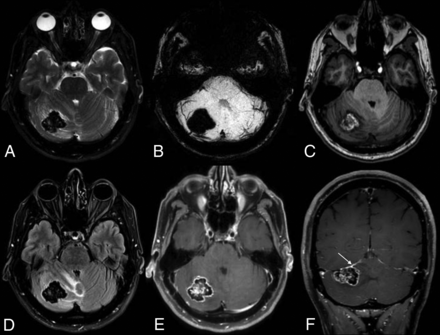
MR imaging at the time of presentation demonstrates a slight interval increase in the size of the mass. Dense intralesional calcifications are again observed, as evidenced by substantial signal drop-out on T2 (A) and susceptibility-weighted (B) images. Axial T1 image (C) shows areas of internal hyperintense signal. FLAIR image (D) shows adjacent vasogenic edema, with a mild associated mass effect on the fourth ventricle. Axial (E) and coronal (F) postcontrast images demonstrate intense peripheral enhancement around the perimeter of the lesion, as well as a possible dural tail (arrow).
The decision was made to proceed to surgical resection on the basis of the presumption that the mass was contributing to the patient's ongoing headaches.
Operative Report and Hospital Course
The mass was resected via a right suboccipital craniotomy. When the surgeon entered the dura, substantial mass effect was observed in the posterior fossa. The tumor was visualized along the superior surface of the cerebellum and was adherent to the tentorium. Because of its solid, chalky consistency, the tumor was removed in a piecemeal fashion. No gross tumor was visible at the completion of the surgery. Postoperatively, the patient developed obstructive hydrocephalus and required placement of an external ventricular drain but did not require permanent CSF diversion. He was discharged to inpatient rehabilitation and made a progressive recovery back to his neurologic baseline.
Pathology
Histologic examination revealed a coarsely calcified mass, in which a chondromyxoid fibrillary matrix was identifiable (Fig 3). While the periphery of the tumor occasionally demonstrated palisading reactive spindle cells and a mild chronic inflammatory infiltrate, the lesion itself was composed of acellular mineralization devoid of a viable cytologic component. The lesion was, therefore, incompatible with a neoplastic entity such as a heavily calcified glioma, which is characterized by a discernible proliferation of cells.
Histologic examination revealed a tumefactive lesion characterized by exuberant calcification (A, original magnification ×50) around areas of chondromyxoid fibrillary matrix (B, original magnification ×20). Palisading spindle cells and a mild chronic inflammatory infiltrate are present on the periphery (C, original magnification ×200). All specimens are stained with hematoxylin-eosin.
Multiple features distinguished this entity from other intracranial masses with a chondromyxoid matrix. Metaplastic meningiomas demonstrate varying degrees of mesenchymal elements that may manifest as myxoid changes, and chordoid meningiomas feature chords and trabeculae of eosinophilic, variably vacuolated cells in a mucoid background reminiscent of chordomas.1,2 Nevertheless, both meningioma variants invariably demonstrate an overt neoplastic cell population, readily differentiating them from the entity observed in this case. In addition, masses exhibiting chondromyxoid elements should be evaluated for the possibility of chondroma and chondrosarcoma. Conventional chondrosarcomas exhibit variable degrees of cellularity, cytologic atypia, and mitotic activity and, similarly, show a distinctive hyaline-to-myxoid cartilage matrix.3 However, the presence of a malignant cytologic component differentiates chondrosarcomas from the current process. The mature hyaline cartilage present in chondromas appears morphologically distinct from the irregular pattern of a chondromyxoid matrix and calcification seen in this lesion.4
In addition, some processes exhibit robust mineralization in the absence of an overt neoplastic cell population, including calcium pyrophosphate disease and myositis ossificans. While calcium pyrophosphate disease may exhibit robust mineralization, it is characteristically composed of rhomboid pyrophosphate crystals that exhibit weak, positive birefringence following examination under polarized light, a quality lacking in the current lesion. Moreover, although myositis ossificans often exhibits prominent calcification, it is, nevertheless, histologically distinctive for having a so-called “zonation” phenomenon, in which progressive maturation of bone is deposited around a central fibroblastic core—features lacking in this process.5
Ultimately, the robust calcification in patchy alternation with the chondromyxoid fibrillary matrix seen in this lesion was most consistent with calcified pseudoneoplasm of the neuraxis (CAPNON). The diagnosis was CAPNON.
DISCUSSION
CAPNONs, also known as fibro-osseous lesions of the CNS, are rare non-neoplastic lesions, first described by Rhodes and Davis,6 in 1978. They are found essentially everywhere in the central nervous system; lesions can be intra- or extra-axial and occur in both the brain and spine.7,8 However, CAPNONs may have a slight predilection for intracranial involvement, specifically occurring along the dura of the skull base.9 The lesions have no predilection for age or sex. Cases have been reported in patients as young as 12 and as old as 90 years of age, with about half of patients presenting between 40 and 60 years of age.9,10 Because symptoms are related to local mass effect, clinical presentations are extremely variable. Headaches, epileptic seizures, cranial nerve dysfunction, and neck/back pain have all been reported; some CAPNONs are discovered incidentally.11⇓-13
The diagnosis of a CAPNON can be strongly suggested by its imaging appearance, especially when intracranial. True to their name, CAPNONs are highly calcific, a feature that is readily apparent on CT. Although lesions are classically described as hypointense on both T1WI and T2WI, T1 hypointensity is not a reliable characteristic, likely due to interactions between calcification and free water molecules, as was seen in the case presented here.14 CAPNONs are usually extra-axial lesions, and dural attachments are observed in most cases. Modest enhancement may or may not be visualized, with either intralesional serpentine or peripheral/rim enhancement patterns.15 Perilesional T2 prolongation, too, is sometimes observed, either representing vasogenic edema or gliosis related to chronic mass effect on the nearby parenchyma.16,17
The imaging differential of CAPNONs mirrors that of other cerebral calculi (“brain stones”). If extra-axial, osteomas and highly calcified meningiomas are the greatest mimickers of CAPNONs.18 Osteomas often develop from the calvaria; thus, intracranial osteomas are often contiguous with the inner table or even the diploic space of the adjacent bone. They also typically lack intracranial enhancement.19 Nevertheless, osteomas can arise from dural attachments, similar to CAPNONs; rarely, some osteomas enhance.20 Large meningiomas are often associated with hyperostosis of the underlying bone, a feature not described in CAPNONs. Meningiomas also usually demonstrate enhancement, though this may be absent in densely calcified lesions. Intra-axial CAPNONs can appear similar to calcified tumors and vascular malformation—particularly oligodendrogliomas and cavernous malformations.21,22 Dystrophic calcifications, too, can share imaging features of CAPNONs, though these typically arise in post-traumatic or postischemic regions of the brain.23
Spinal lesions tend to be smaller and less characterizable than their intracranial counterparts. The large majority of spinal CAPNONs (>80%) are in the epidural space.10 Garcia Duque et al10 found that the cervical region was the most frequent location of spinal CAPNONs. Other studies, however, have suggested otherwise; a large single-institution study by Ho et al14 noted that most spinal CAPNONs were in the lumbar spine. Calcified psammomatous meningiomas, calcium pyrophosphate dihydrate deposition disease, exophytic osseous lesions, synovial cysts, calcified hematomas, and herniated discs would be the most likely alternative diagnostic considerations.24
Histologically, CAPNON is typically characterized as a process exhibiting robust calcification in a patchy, nonzonal alternation with chondromyxoid fibrillary matrix. While a chondromyxoid fibrillary matrix has been classically described as a defining feature of this entity, a recent case series has noted that calcification and cartilaginous elements are more consistent features across patients.14 While the lesion may incite reactive meningothelial cells and macrophages around the periphery of the lesion, these cellular elements should not be an underlying substrate of the lesion itself.
The etiology of CAPNONs remains uncertain. However, both their histologic characteristics and behavior have led to the presumption that CAPNONs are reactive entities.18 The source of the reactive process can vary, ranging from inflammation to trauma. Ho et al14 found that nearly one-third of CAPNONs were so-called “collision” lesions, in which CAPNON tissue coexisted with a separate, distinct pathologic entity. The coexistent primary tumors found in such collision lesions included meningiomas, pleomorphic xanthoastrocytomas, dysembryoplastic neuroepithelial tumors, and vascular malformations. Dual lesions have similarly been reported elsewhere.8,25 Histologic analyses of collision lesions have demonstrated inflammatory tissue outside the CAPNON boundaries, suggesting that the primary lesions initiated a reactive process, ultimately resulting in the formation of a CAPNON.14 In the spine, too, associated pathologies are also observed, including synovial cysts and degenerated discs.
The management and prognosis of CAPNONs are highly dependent on lesion location. Total resection tends to be curative, though exceptionally rare cases have been reported in which recurrence or progression of lesions or both occur after incomplete resection.11 Still, surgical removal can be particularly challenging when the lesion is located within eloquent regions because piecemeal resection is often required due to the dense intralesional calcifications.26
Case Summary
CAPNONs are non-neoplastic lesions, found anywhere within the CNS and can appear as intra- or extra-axial masses. Although extremely rare, the diagnosis of a CAPNON can be highly suggested on the basis of its imaging appearance, particularly when large and intracranial. Histologically, a CAPNON is typically characterized as a process exhibiting robust calcification with cartilaginous elements and may exhibit a characteristic chondromyxoid fibrillary matrix. Prognosis tends to be excellent, and surgical resection is curative.
References
- Received April 6, 2021.
- Accepted after revision May 4, 2021.
- © 2021 by American Journal of Neuroradiology