Abstract
BACKGROUND AND PURPOSE: Accurate differentiation between glioblastoma and solitary brain metastasis is of vital importance clinically. This study aimed to investigate the potential value of the inflow-based vascular-space-occupancy MR imaging technique, which has no need for an exogenous contrast agent, in differentiating glioblastoma and solitary brain metastasis and to compare it with DSC MR imaging.
MATERIALS AND METHODS: Twenty patients with glioblastoma and 22 patients with solitary brain metastasis underwent inflow-based vascular-space-occupancy and DSC MR imaging with a 3T clinical scanner. Two neuroradiologists independently measured the maximum inflow-based vascular-space-occupancy–derived arteriolar CBV and DSC-derived CBV values in intratumoral regions and peritumoral T2-hyperintense regions, which were normalized to the contralateral white matter (relative arteriolar CBV and relative CBV, inflow-based vascular-space-occupancy relative arteriolar CBV, and DSC-relative CBV). The intraclass correlation coefficient, Student t test, or Mann-Whitney U test and receiver operating characteristic analysis were performed.
RESULTS: All parameters of both regions had good or excellent interobserver reliability (0.74∼0.89). In peritumoral T2-hyperintese regions, DSC-relative CBV (P < .001), inflow-based vascular-space-occupancy arteriolar CBV (P = .001), and relative arteriolar CBV (P = .005) were significantly higher in glioblastoma than in solitary brain metastasis, with areas under the curve of 0.94, 0.83, and 0.72 for discrimination, respectively. In the intratumoral region, both inflow-based vascular-space-occupancy arteriolar CBV and relative arteriolar CBV were significantly higher in glioblastoma than in solitary brain metastasis (both P < .001), with areas under the curve of 0.91 and 0.90, respectively. Intratumoral DSC-relative CBV showed no significant difference (P = .616) between the 2 groups.
CONCLUSIONS: Inflow-based vascular-space-occupancy has the potential to discriminate glioblastoma from solitary brain metastasis, especially in the intratumoral region.
ABBREVIATIONS:
- AUC
- area under the curve
- CBVa
- arteriolar CBV
- GBM
- glioblastoma
- iVASO
- inflow-based vascular-space-occupancy
- rCBV
- relative CBV
- rCBVa
- relative arteriolar CBV
- PTH
- peritumoral T2-hyperintesity region
- SBM
- solitary brain metastasis
Glioblastoma (GBM) accounts for 40%∼50% of all primary malignant brain tumors in adults. Brain metastases are the most common complication of systemic cancer, and half of them are solitary at diagnosis.1 It is clinically important to distinguish GBM from solitary brain metastasis (SBM) because of the vast differences in these 2 entities with regard to tumor staging, treatment approach, and clinical outcomes.2-4 Structural gadolinium-enhanced MR imaging is the preferred imaging examination for brain tumors, but with a limited capacity to differentiate GBM and SBM. They share similar imaging features, such as extensive edema and ring-enhancement, which is a great challenge in clinical practice.5-7
Many studies have demonstrated that PWI is a promising technique to discriminate GBM from SBM, due to its capability to disclose the differences between them in angiogenesis and vascularity.8,9 In particular, DSC MR imaging is the most robust perfusion technique to perform such a task.10,11 However, most studies found that DSC-derived relative CBV (rCBV) in intratumoral regions does not permit reliable differentiation between high-grade gliomas and metastases,1,6,12⇓⇓-15 which was thought to be related to contrast leakage from tumor vessels and, consequently, unreliable estimation of CBV.10,16,17 rCBV measured in peritumoral regions may be effective in this regard, but this method inherently has some major disadvantages due to indefinite tumoral boundary and various definitions of the peritumoral area.15,18 Besides, the administration of exogenous contrast agents required for DSC raises concerns about the adverse effects of gadolinium, especially the deposits in brain, even using macrocyclic gadolinium-based contrast agents.19⇓⇓-22
Inflow-based vascular-space-occupancy (iVASO) is a completely noninvasive perfusion method that does not involve administration of an exogenous contrast agent.23 Instead, proton spins in the water molecules in blood are exploited as an endogenous contrast agent by applying spatially selective radiofrequency inversion pulses.24 iVASO emphasizes the perfusion blood volume in arteries and arterioles. The absolute CBV of pial arteries and precapillary arterioles (arteriolar CBV [CBVa]) can be calculated from the different signals between a scan with arterial blood signal selectively zeroed out (nulled) and a control scan without blood nulling.24,25 Notably, previous studies have demonstrated that pial arteries and arterioles are the most sensitive vessels to respond via adaptive hemodynamic adjustment to changes in cerebral metabolism status in the human body.26-28 According to recent studies, CBVa measured with iVASO MR imaging (iVASO-CBVa) has proved sensitive in disclosing microvascular abnormalities in the early stage of some mental and cognitive disorders, such as Huntington disease, Alzheimer disease, and schizophrenia.29-31 Furthermore, a previous study has demonstrated that iVASO-CBVa is strongly correlated with glioma grades and might be superior to DSC-derived rCBV.32 Therefore, we hypothesized that iVASO can distinguish GBM from SBM. To validate this hypothesis, we performed iVASO MR imaging on patients with GBM or SBM on a clinical 3T MR imaging scanner.
MATERIALS AND METHODS
Study Participants
This retrospectively reviewed study was prospectively controlled and conducted between December 2015 and March 2017. All examinations were performed in accordance with institutional (Nanfang Hospital Southern Medical University) review board guidelines with an approved study protocol. Inclusion criteria were as follows: 1) patients with a single, solitary enhancing brain mass with a clinical question of SBM versus GBM; 2) pretreatment acquisition of a 3T MR imaging brain tumor protocol, including structural MR imaging, iVASO, and DSC; and 3) the mass pathologically confirmed by stereotactic biopsy or surgical sample within 2 weeks after MR imaging. Ten patients were excluded (4 for obvious movement artifacts, 4 for susceptibility artifacts hampering ROI placement, 2 for small lesions greatly influenced by partial volume averaging effect). The remaining 20 patients with GBM and 22 with SBM were eventually included in the study.
MR Imaging Acquisition
MR imaging examinations were implemented with a clinical 3T imaging unit (Achieva 3T; Philips Healthcare, Best, the Netherlands) equipped with an 8-channel head coil. DSC, iVASO, and structural MR imaging were performed for each subject in the same scan session.
The structural MR imaging protocol included an axial FLAIR scan (TR/TI/TE = 11,000/2200/125 ms, voxel = 0.7 × 0.7 × 6 mm3, 20 slices), T2WI (TR/TE = 3000/80 ms, voxel = 0.5 × 0.7 × 6 mm3, 20 slices), and T1WI (TR/TE = 2000/20 ms, voxel = 0.5 × 0.9 × 6 mm3, 20 slices) (detailed in On-line Table 1). Contrast-enhanced fat-suppressed T1WI (TR/TE = 297/4.6 ms, voxel = 0.5 × 0.7 × 6 mm3, 20 slices) was obtained after DSC.
3D iVASO was performed before contrast agent administration. Parameters for the iVASO pulse sequence were the following: TR/TI = 5000/1040, 3100/862, 2500/756, 2000/641, 1700/558, 1300/430 ms; 3D gradient spin-echo readout (TE = 10 ms; voxel = 2.5 × 2.5 × 6 mm3, 14 slices); parallel imaging acceleration (sensitivity encoding) = 2 × 2; crusher gradients of b = 0.3 s/mm2 and Venc = 10 cm/s along the z-direction. A reference scan (TR = 20 seconds, other parameters identical) was obtained to determine the scaling factor M0 in iVASO images so that absolute CBVa values could be calculated. The total duration of all iVASO-related scans combined was 7 minutes for each patient.
DSC perfusion images were acquired immediately after contrast agent injection with fast-field echo, echo-planar imaging, TR/TE = 1700/40 ms, FOV = 210 × 210 mm, pixel = 2.3 × 2.3 mm, matrix = 90 × 90, thickness/gap = 6/0 mm, 20 slices, 60 dynamics, flip angle = 75°. Contrast agent (gadodiamide, Omniscan; GE Healthcare, Piscataway, New Jersey) was administered at a dose of 0.2 mmol/kg and a rate of 4.5 mL/s, using a power injector (Spectris Solaris EP; MedRad, Indianola, Pennsylvania) through the antecubital vein, followed by a 20- mL sterile saline flush at the same rate. The total acquisition time of DSC was 1 minute 42 seconds.
MR Imaging Analysis
All iVASO data were preprocessed using the Statistical Parametric Mapping software package (Version 8; http://www.fil.ion.ucl.ac.uk/spm/software/spm12). The iVASO images were analyzed with in-house routines programmed in Matlab (MathWorks, Natick, Massachusetts), which were used for a previously published report.32 DSC images were processed on an Advantage Workstation using FuncTool (Version 4.6; GE Healthcare) to obtain CBV maps.
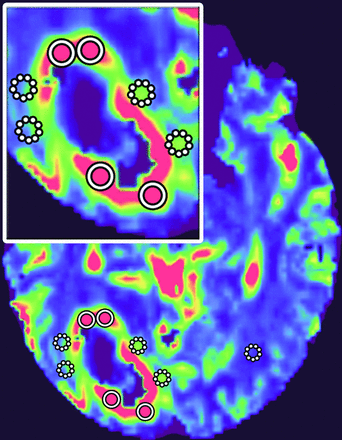
Color-coded iVASO-CBVa and DSC-CBV maps were generated, respectively. The region of maximal abnormality of each parameter within the lesion volume (hotspot) was determined via visual inspection. This methodology was demonstrated to provide the most optimal interobserver and intraobserver reproducibility.33 Four ROIs of about 20 pixels were carefully placed on the hotspots, respectively, in the intratumoral region and the peritumoral T2-hyperintense region (PTH), to obtain the maximum iVASO-CBVa and CBV of each region. The PTH was defined as the T2-hyperintense region within 1 cm around the enhancing tumor.13 ROIs were drawn in the contralateral white matter as references for normalization (iVASO-rCBVa and DSC-rCBV) (Fig 1). All ROIs were placed independently by 2 blinded experienced neuroradiologists (X. Li and Y. Wu, with 5 and 12 years of experience, respectively). The measurement results of the 2 radiologists were used to assess the interobserver reliability. The average of the 2 measurement results was used for further statistical analysis.
ROI placements. Four to six ROIs were drawn in both intratumoral (circle with solid line) and peritumoral (circle with dotted line) regions, and the maximum value was recorded. Also, an ROI in the contralateral white matter (circle with dotted line) was chosen as a reference. The insert is the magnification of lesion area.
Statistical Analysis
Statistical analysis was performed using SPSS Statistics 22.0 (IBM, Armonk, New York). Interobserver reliability of the parameters between the 2 neuroradiologists was assessed by the intraclass correlation coefficient with 95% confidence intervals. Intraclass correlation coefficient values of ≤0.40, between 0.41 and 0.60, between 0.61 and 0.80, and ≥0.81 were interpreted as poor, moderate, good, and excellent reliability, respectively. iVASO-CBVa, rCBVa, and DSC-rCBV in intratumoral and peritumoral regions were correlated with each other by calculating Pearson correlation coefficients. The Shapiro-Wilk test was used to assess the normality of data distribution. Comparisons between the GBM and SBM groups were performed using the Student t test or Mann-Whitney U test accordingly. Receiver operating characteristic and area under the curve (AUC) were used to assess the diagnostic value of parameters for discrimination. An area under the receiver operating characteristic curve greater than 0.90 was considered excellent; 0.80–0.90 was considered good; 0.70–0.80 was considered fair; 0.60–0.70 was considered poor; and <0.50 was considered a failure. The cutoff value was established by maximizing the Youden index (Youden index = sensitivity + specificity –1). A statistical significance of a P value < .05 was used.
RESULTS
Twenty GBMs (16 men and 4 women, with a mean age of 46.1 years; range, 18–62 years), which included 19 patients without the isocitrate dehydrogenase 1 (IDH1) mutation and 1 with the IDH1 mutation, and 22 SBMs (13 men and 9 women, with a mean age of 56.6 years; range, 44–65 years), which included 18 patients with primary non-small cell lung adenocarcinoma, 2 with breast adenocarcinoma, 1 with prostate cancer, and 1 with hepatocellular carcinoma, finally met all of our inclusion and exclusion criteria. Among them, 2 patients with GBMs and 17 with SBMs were diagnosed on the basis of stereotactic biopsy samples, and the conditions of the others were confirmed by gross total resection.
The interobserver reliability was excellent for iVASO-CBVa in PTH (intraclass correlation coefficient = 0.82) and was good for DSC-rCBV (intraclass correlation coefficient = 0.80) and iVASO-rCBVa (intraclass correlation coefficient = 0.74). In the intratumoral region, all the parameters demonstrated excellent reliability (intraclass correlation coefficient = 0.86∼0.89).
The results of correlation analysis are given in On-line Table 2. In the intratumoral region, no substantial correlation was observed between iVASO-CBVa or rCBVa and DSC-rCBV (P = .23 and .18), while in the peritumoral region, a mild correlation was observed between iVASO-CBVa or rCBVa and DSC-rCBV (P < .001).
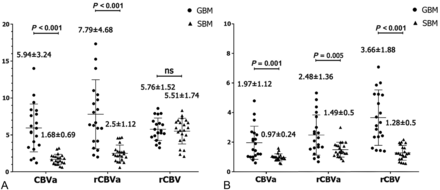
Perfusion values are plotted in Fig 2. In intratumoral regions, both iVASO-CBVa and rCBVa were significantly higher in patients with GBM than in those with SBM (P = .001 and .005, respectively), while DSC-rCBV showed no significant difference between them (P = .616). In PTH, DSC-rCBV, iVASO-CBVa, and rCBVa revealed higher values in GBM than in SBM (P < .001). Representative cases including iVASO and DSC perfusion MR images are shown in Fig 3.
iVASO-CBVa, rCBVa, and DSC-rCBV in the intratumoral region (A) and in peritumoral T2-hyperintense region (B) of glioblastoma and single brain metastasis. Data are presented as mean value ± SD. Ns indicates not significant.
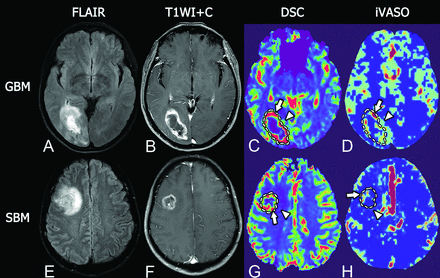
Representative MR images of glioblastoma and single brain metastasis. Upper row, A GBM in a 51-year-old woman. Lower row, SBM in a 46-year-old man. Both GBM and SBM present as hyperintense masses on T2 FLAIR with extensive peritumoral edema (A and E) and show a ring-enhancement pattern on fat-suppressed postcontrast T1WI with prominent necrosis in the tumor center (B and F). In intratumoral regions, GBM shows maximum DSC-rCBV similar to that of SBM (5.29 versus 4.98, arrows in C and G), but higher maximum iVASO-CBVa than SBM (5.90/100 mL versus 1.30/100 mL, arrows in D and H). In the peritumoral region, GBM shows prominently higher DSC-rCBV and iVASO-CBVa than SBM (DSC-rCBV, 3.11 versus 1.25, arrowheads in C and G; iVASO-CBVa, 1.20/100 mL versus 0.55/100 mL, arrowheads in D and H).
The results of receiver operating characteristic analysis of each parameter for differentiating GBM and SBM are listed in the Table and plotted in Fig 4. In intratumoral regions, both iVASO-CBVa and rCBVa showed excellent performance, with AUCs of 0.91 and 0.90, a sensitivity of 80% and 70%, and a specificity of 100% and 100%, respectively, which was comparable with that of DSC-rCBV in PTH (AUC, 0.94; sensitivity, 80%; specificity, 100%). In PTH, the AUCs of iVASO-CBVa and rCBVa were 0.83 and 0.72, respectively.
Results of receiver operating characteristic analysis of each parameter
Receiver operating characteristic curves of parameters of iVASO and DSC MR imaging in differentiating glioblastoma and solitary brain metastasis. In the intratumoral region (A), both iVASO-CBVa (AUC = 0.91) and rCBVa (AUC = 0.90) show higher AUCs than DSC-rCBV (AUC = 0.51). In the PTH (B), the AUC of DSC-rCBV (0.94) is higher than that of iVASO-CBVa (0.83) or rCBVa (0.72).
DISCUSSION
Reliable differentiation between GBM and SBM is of vital clinical importance. Our preliminary study investigated the capacity of iVASO MR imaging to differentiate these 2 types of tumors. The results showed that iVASO-derived CBVa and rCBVa in both intratumoral and peritumoral PTH can accurately discriminate GBM and SBM and that DSC-rCBV was powerful for the discrimination between them only in PTH.
Within PTH, DSC-rCBV can accurately discriminate GBM from SBM according to our study, which was concordant with previous reports.5,14,15 This finding may be explained by the obviously different perfusion values in the PTH between these entities. Histopathologically, GBM tends to grow in an invasive manner and extends to the PTH beyond the contrast-enhancing margins.34-39 On the contrary, SBM tends to grow in an expansile way, leading to no prominent infiltration of tumor cells in the PTH beyond the area of contrast enhancement.15,35,40 However the definition of the tumor boundary is a common issue of controversy within this field. Researchers have defined the tumoral and peritumoral areas in various ways.1,13,15,41 For gliomas, the so-called peritumoral regions pathologically consist of benign changes, such as vasogenic edema and inflammatory reaction, as well as infiltration by tumor cells. Besides, the peritumoral edema areas of GBM and metastasis are usually extensive and may include different lobes and even extend to the whole cerebral hemisphere and the contralateral hemisphere. This feature will make drawing the ROI relatively difficult and thus affect the interobserver reliability,42 as shown in our present study in which the interobserver reliability of the peritumoral region (0.74∼0.82) was lower than that of the intratumoral region (0.86∼0.89).
According to most previous studies, the intratumoral region is the mainstream region for measurement.43,44 The intratumoral perfusion is closely related to tumor biologic characteristics, gene mutation status, therapeutic response, and prognosis.45-48 Unfortunately, most of these studies have demonstrated that intratumoral DSC-rCBV was not powerful for differentiating GBM from SBM,1,6,12-15 as shown in our study. Of note, this finding does not mean that these 2 types of tumors share the same characteristics of microvasculature. Weber et al13 observed significantly larger microvessel density in GBM than in brain adenocarcinoma metastases. According to the study of Jinnouchi et al,49 the capillaries of brain metastasis resemble those from the site of the original systemic cancer and thus have no similarity to the normal brain capillaries and completely lack BBB components. On the other hand, GBM is primary brain tumor and has a blood-brain barrier, albeit a heterogeneous, disrupted one.8,50 Lai et al51 and Fu et al52 reported that the degree of intralesional susceptibility signal was significantly higher in GBM than in SBM. Intralesional susceptibility signal reflects the conglomerates of tumor microvascularity, and the degree of intralesional susceptibility signal showed a significant correlation with the value of maximum DSC-rCBV in the same tumor segments.44,53,54 Furthermore, a few investigators reported significant perfusion differences between GBM and SBM, using parameters of peak height or percentage signal recovery35,55,56 or histogram analysis of rCBV.57
In the present study, both iVASO-CBVa and rCBVa accurately differentiated GBM from SBM and outperformed DSC-rCBV without leakage correlation via assessment of the intratumoral regions. Interobserver reliability analysis demonstrated poor reliability between iVASO-derived parameters and DSC-rCBV in intratumoral regions. This may be mainly due to the different compartments assessed by iVASO and DSC. iVASO is designed to quantify the blood volume of the arterioles, while DSC quantifies the perfusion of the whole microvasculature.23,24 Physiologically, arterioles and pial arteries are the most actively regulated blood vessels in the microvasculature.27,58,59 They control the cerebral perfusion of the whole microvasculature unit through the contraction and relaxation of smooth-muscle and elastic lamina.60,61 Also, there is evidence that generation of arterioles occurs before capillary growth in angiogenesis.62 The predominant arterial origin of the iVASO signal was validated in a previous study by measuring the transverse relaxation times (T2*/T2) of iVASO difference signals, which are highly oxygenation-level dependent.63 Besides, the iVASO signal changes during functional stimulation, such as somatosensory stimulus and forepaw stimulation, preceded the changes in total CBV,24 which corresponded to animal studies showing earlier changes in arterioles upon neuronal activation.64,65 Therefore, the ability to measure arteriolar CBV separately from the rest of the microvasculature (capillaries and venous vessels) may furnish information that is not obtainable from total CBV measures (ie, DSC-CBV) and may make the measurement more sensitive in reflecting hemodynamics changes.31 Notably, the wall of the arterioles is not permeable to magnetically labeled spin protons. Hence, the CBVa value would not be affected by the disrupted blood-brain barrier. In contrast, the measurement of DSC-rCBV is remarkably confounded by the disrupted blood-brain barrier. Our results demonstrated significant differences in iVASO-CBVa between GBM and SBM. This finding indicates the difference in the arteriolar compartment between them, which is in line with the results revealed by previous studies.8,13,49⇓⇓-52
Most interesting, intratumoral iVASO-CBVa had a diagnostic value approximate to that of iVASO-rCBVa. This finding may suggest that the discrimination between GBM and SBM can be achieved by measuring the perfusion value within the intratumoral region alone, which will enhance the clinical applicability of iVASO, whereas iVASO-CBVa and rCBVa in the PTH showed lower capability than their intratumoral counterparts in distinguishing these 2 groups of tumors. This might be related to the heterogeneity and complexity of the microenvironment in the PTH. Also, according to the theory of iVASO, the measurement of iVASO-CBVa is based on the arterial transit time of gray matter, so it is sensitive to blood flow with a relatively high speed.23 However, the arterial transit time in white matter is relatively long, which will reduce the sensitivity of iVASO in quantifying perfusion.24,66,67 In contrast, DSC is designed to mainly quantify the capillary bed, so it will not be affected by the relatively slow blood flow in the white matter regions.32
DSC MR imaging, the most widely used perfusion MR imaging technique, was recommended as a routine protocol by the 2018 European Guidelines in brain tumor MR scanning.11 However, the deposit of exogenous contrast agent of gadolinium is a major issue of public concern.19⇓⇓-22 Also, logistically, the inconvenience of the bolus injection of contrast agent in children and elderly patients has limited the application of DSC scanning.32,67 Besides, according to most studies,1,12 DSC failed to discriminate GBM from SBM via analysis of the intratumoral region, just as shown in our present study. iVASO is a totally noninvasive perfusion technique without the need for exogenous contrast agents. Of note, the actual scan time of iVASO MR imaging is usually several minutes longer than DSC MR imaging. However, one important practical advantage of iVASO is that the perfusion data can be obtained in a flexible manner and can be integrated into a conventional MR imaging examination at any time as long as no contrast agent has been administered.32,67
Our study has several limitations. First, the sample size is relatively small. Therefore, a larger cohort study is needed to validate these results in the future. Also, metastases of other different cancer subtypes were not included in our study. Second, we did not use histogram analysis to study the tumor perfusion. Generally, histogram analysis reveals more objective results. However, the additional time-consuming postprocessing involved may lower its clinical practicability. Considering that the hotspot method showed good reliability, we believed that this would not essentially affect our main results. Moreover, we did not apply the leakage-correction analysis method or preload gadolinium-based contrast agent in our DSC protocol to reduce the variance of gadolinium rCBV estimates.68 However, a recent DSC study applying preload of contrast agent failed to discriminate these 2 groups of tumors in the intratumoral region.12 Moreover, the complexity of the operation and the consumption of more time, which greatly hinder patient compliance and cause motion artifacts, limit the use of leakage-correction and preload strategy in clinical practice.69 In addition, it would be better to perform imaging-pathology correlation analysis. However, because the section thickness (6 mm) of the current iVASO technique is inferior to the requirement for stereotactic biopsy, one-to-one correspondence between the biopsied regions and imaged regions is not possible.
CONCLUSIONS
This preliminary study demonstrated that iVASO might be useful for discriminating GBM from SBM based on the analysis of either PTH or intratumoral region. Due to its completely noninvasive nature, iVASO might greatly benefit patients with brain tumor in daily clinical practice, especially for elderly populations and those with compromised renal function.
Footnotes
Xiaodan Li and Danni Wang contributed equally to this study.
This study has received funding from the Natural Science Foundation of Guangdong Province, China (grant No. S201301005689), the Science and Technology Program of Guangzhou, China (grant No. 201707010003), and the Special Foundation of President of Nanfang Hospital, Southern Medical University (grant No. 2016B026).
Disclosures: Jay Pillai—UNRELATED: Royalties: Springer Science & Business Media, Elsevier, Comments: royalties for books published.
Abstract previously presented orally at: Annual Meeting and Exhibition of the International Society for Magnetic Resonance in Medicine, May 11–16, 2019; Montreal, Quebec, Canada.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received July 23, 2019.
- Accepted after revision February 3, 2020.
- © 2020 by American Journal of Neuroradiology