Abstract
BACKGROUND AND PURPOSE: Idiopathic intracranial hypertension is a syndrome of raised intracranial pressure of unknown etiology. Few MR imaging–based studies have investigated arterial and venous blood flow in these patients. Results are inconclusive, and to our knowledge, no comparison of the hemodynamic parameters before and after CSF pressure reduction has been published. The aim of this study was to assess the short-term effects of normalizing CSF pressure on intracranial flow to better understand the pathophysiology of idiopathic intracranial hypertension.
MATERIALS AND METHODS: In this study, we performed quantitative MR imaging–derived flow measurements of brain-supplying arteries and draining veins/dural sinuses to visualize hemodynamic changes in patients with idiopathic intracranial hypertension before and after therapy by lumbar puncture in comparison with a healthy control group.
RESULTS: We found differences in patients before and after lumbar puncture in the calculated resistance and pulsatility indices in the superior sagittal sinus. Venous pulsatility showed a negative correlation with CSF pressure in untreated patients. Additionally, there was a trend toward lower flow in the superior sagittal sinus in patients compared with healthy controls. Flow in the internal jugular veins was significantly reduced by lumbar puncture, and the resistance and pulsatility indices differed in patients and controls. The arterial flow was not influenced by pressure normalization.
CONCLUSIONS: The results of the present study indicate that venous but not arterial blood flow differs in patients compared with controls and that calculating resistance and pulsatility indices may contribute to assessing short-term hemodynamic changes in patients with diagnosed idiopathic intracranial hypertension before and after CSF diversion.
ABBREVIATIONS:
- BA
- basilar artery
- IIH
- idiopathic intracranial hypertension
- IJV
- internal jugular veins
- LP
- lumbar puncture
- PI
- pulsatility index
- RI
- resistance index
- SS
- straight sinus
- SSS
- superior sagittal sinus
Idiopathic intracranial hypertension (IIH), also known as pseudotumor cerebri, is a syndrome of raised intracranial pressure of unknown etiology.1 Clinical symptoms include headache, visual impairment, nausea, and papillary edema.2,3 The initial diagnosis is based on clinical assessment, evidence of increased CSF pressure as per direct measurement (ie, lumbar puncture), and cranial imaging excluding mass lesions and venous sinus thrombosis.4⇓–6 The finding of transverse sinus stenoses in most patients with IIH, easily visualized with MR venography, has raised the question of whether IIH is caused by (or results in) impaired cranial blood flow, and studies using MR imaging flow techniques indicate that arterial hyperperfusion and abnormal venous outflow are present in patients with IIH.7,8 Reducing intracranial pressure in patients with IIH may interfere with a pathologic feedback loop, in which a focal collapse of dural sinuses maintains CSF pressure at an increased level, with increased CSF pressure perpetuating collapse of the dural sinuses. To our knowledge, the short-term effects of normalization of intracranial pressure using lumbar puncture on intracranial flow properties have not been systematically studied. Our study compares MR imaging–derived blood flow properties in patients with IIH before and after normalization of CSF pressure and in healthy controls and aims to answer 2 questions: Can MR imaging flow studies help identify patients with IIH compared with healthy controls? What are the short-term effects of normalizing CSF pressure on intracranial flow (ie, does flow return to normal with treatment)?
Materials and Methods
Subjects
Twenty consecutive patients seen in the Department of Radiology and Neuroradiology of the UKSH Kiel (18 women, 2 men; mean age, 34.4 ± 13.8 years; mean body mass index, 35.9 ± 8.9 kg/m2) with a clinical diagnosis of IIH were included in the study and underwent MR imaging before lumbar puncture (LP). All patients gave written informed consent before scanning, and the study was approved by the local ethics committee. All patients had signs and symptoms of raised intracranial pressure in the presence or absence of prior medical treatment, in particular visual disturbances related to papilledema. LP revealed opening CSF pressures from 21 to >50 cm H2O (mean, 35 cm H2O) and normal CSF composition. Patients with secondary or unconfirmed disease (ie, normal CSF pressure) were excluded. The mean CSF drained was 31.3 ± 5.3 mL, and the pressure was reduced to 10.4 ± 9.9 cm H20. After LP, a second MR imaging was performed on the same day.
The control group consisted of 20 volunteers (17 women, 3 men; mean age, 34.9 ± 11.2 years) without neurologic disease, undergoing a single MR imaging examination. None of the controls had signs or symptoms of IIH, and none of them had sinus stenosis.
MR Imaging and Analysis
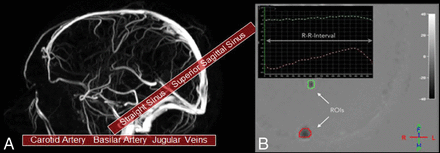
MR imaging was performed on a 1.5T Achieva scanner (Philips Healthcare, Best, the Netherlands) using a 6-channel receive head coil. To exclude secondary causes of the symptoms, we conducted routine MR imaging, including diffusion-weighted imaging, T2-weighted imaging, and T2-weighted fluid-attenuated inversion recovery. To assess dural sinus morphology, we used 3D phase conventional angiography. To measure venous and arterial hemodynamic properties, we used retrospective 2D cardiac-triggered phase-contrast angiography. Four single-slice measurements were performed. Two were positioned across the neck to evaluate the internal jugular veins and the internal carotid arteries. The others were positioned to image the straight sinus (SS), the superior sagittal sinus (SSS), and the basilar artery (BA). Slice positioning is demonstrated in Fig 1A. Velocity-encoding was set to 40 and 100 cm/s to image venous and arterial flow, respectively. Imaging parameters for phase-contrast angiography were the following: fast-field echo imaging with 2.2 × 2.2 mm voxel size; 20-mm slice thickness; FOV, 200 ×165 mm; flip angle, 15°; TR/TE, 4.6/3.0 ms (venous) and 4.0/2.5 ms (arterial); sensitivity encoding factor, 2. The electrocardiography trigger had a tolerance of +10% and −20% per heartbeat.
A, Slice positioning of the quantitative phase-contrast angiography measurements. Each slab was acquired twice, first with a velocity-encoding of 40 cm/s to visualize venous outflow and then with 100 cm/s to resolve arterial inflow. B, Placement of the measurement ROIs to measure flow properties in the superior sagittal sinus (red circle) and the straight sinus (green circle) with the corresponding measurement points in 1 R-R interval.
Image analysis was conducted by ROI analysis using QFlow (Philips Healthcare). ROIs were placed by an experienced neuroradiologist (J.J.) to cover the area of the BA, SS, and SSS as well as the left and right ICAs and internal jugular veins (IJV). A representative example is shown in Fig 1B. Total arterial inflow was calculated by adding the flow in the BA to the flow in both ICAs. Flow in the IJV represents total venous outflow. The resistance index (RI) and pulsatility index (PI) of blood flow in all vessels were determined according to the following formulas:

 where vsystolic describes the maximum systolic velocity; vdiastolic, the minimum diastolic velocity; and vmean, the mean velocity. Venous sinus morphology was assessed by a senior neuroradiologist (A.R.) on the basis of standard phase-contrast imaging, with distinct transverse sinus stenoses assumed when the diameter of the transverse sinus was focally reduced to ≥50% on both sides, compared with the normal-appearing adjacent sinus diameter, and indistinct narrowing when there was <50% reduction in diameter or only unilateral narrowing. Technical parameters were the following: TR/TE, 17/7.9 ms; flip angle, 10°; FOV, 220 × 220; velocity-encoding, 20 cm/s.
where vsystolic describes the maximum systolic velocity; vdiastolic, the minimum diastolic velocity; and vmean, the mean velocity. Venous sinus morphology was assessed by a senior neuroradiologist (A.R.) on the basis of standard phase-contrast imaging, with distinct transverse sinus stenoses assumed when the diameter of the transverse sinus was focally reduced to ≥50% on both sides, compared with the normal-appearing adjacent sinus diameter, and indistinct narrowing when there was <50% reduction in diameter or only unilateral narrowing. Technical parameters were the following: TR/TE, 17/7.9 ms; flip angle, 10°; FOV, 220 × 220; velocity-encoding, 20 cm/s.
Results of flow measurements were compared using a paired t test for the patient group before and after lumbar puncture. A nonpaired t test was used to assess differences between the patient group before LP and the control group. A P value < .05 was considered statistically significant.
Results
Arterial Inflow
Total arterial inflow values demonstrated no significant differences in patients before versus after LP and between the patient group and the control group, nor did the RI and PI values obtained in the ICA and BA demonstrate any. Flow in the ICAs was higher and flow in the basilar artery was lower in patients than in controls, but the flow did not change after LP.
Venous Outflow
Flow values in the SSS in patients before and after LP were lower than in healthy controls, but differences reached statistical significance only for patients after LP. Flow did not change significantly after LP. RI and PI in the SSS were lower in patients compared with controls as well as after LP. PI showed a negative correlation with CSF pressure in untreated patients.
Flow in the IJV did not differ between patients and controls but was significantly reduced after LP in patients. RI and PI in the IJV were lower in patients before and after LP compared with controls. Again, RI and PI were lower after LP. In the straight sinus, no differences were observed among all groups regarding flow, RI, and PI.
Dural Sinus Stenoses
Seventeen of 20 patients (85%) showed distinct bilateral transverse sinus narrowing on MR venograms, with the remaining 3 patients showing only mild-to-moderate narrowing. After LP, sinus narrowing normalized in 2 patients, improved in another 4 patients, but was unchanged in 11/20 patients. None of the controls showed distinct sinus narrowing.
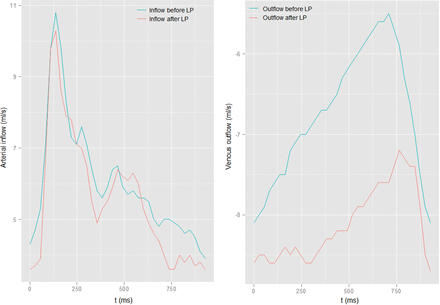
In Table 1, a list of the most important results is presented. The results from the remaining measurements are listed in the Tables 2 and 3. Figure 2 demonstrates an example of a patient with IIH before and after LP.
Results of flow measurements, RI, and PI in the intracranial vasculature of n = 20 patients and n = 20 controlsa
Results of flow measurements, RI, and PI in the intracranial vasculature of n = 20 patients and n = 20 controlsa
Corresponding P values for Table 2
Example of the flow measurements (carotid arteries) of 1 patient showing no difference before and after LP (left). On the venous measurements of the SSS (right), a change in flow can be observed.
Discussion
In recent years, the value of cranial MR venography has been investigated for its potential in diagnosis and monitoring therapeutic success in patients with IIH.9 While the presence of bilateral transverse sinus stenoses was found to be a strong predictor of IIH by several authors, follow-up studies are rare and have yielded conflicting results.10 In some cases, resolution of sinus narrowings was observed after normalization of intracranial pressure, but others reported that sinus stenoses persisted in most patients who improved clinically on follow-up.10⇓⇓–13 It is, however, largely accepted that sinus narrowing in patients with IIH plays a role in increasing intracranial venous and intracranial CSF pressure, and interesting theoretic models have been proposed explaining the underlying mechanisms.14⇓–16 The pathophysiology of IIH is not well-understood, and several hypotheses to explain the etiology of this disease have been published.17 Of particular interest is a model that explains increased CSF pressure being induced by a negative feedback loop: A self-limiting collapse of the transverse sinuses is presumed to result in a different equilibrium state with an increase in intracranial venous and CSF pressure, depending on the compliance of the sinus wall.18
Also, several case series have reported the success of dural sinus stent placement in these patients, further confirming the importance of dural sinus stenoses in the pathophysiology of IIH.19,20 Intravascular MR imaging–based flow studies in patients with IIH could therefore be of interest in elucidating exactly which hemodynamic changes occur and have been conducted by a few authors. Hemodynamic properties studied include the total inflow and outflow rate (milliliters/minute) and flow velocity (centimeters/second) of the intracranial arterial and venous systems, respectively.7,8 From these values, the resistance and pulsatility indices can be derived. MR imaging phase conventional angiography, also used in this study, is an established technique with reported error rates below 5% and a negligible intra- and interobserver variability.21⇓–23 Results by Bateman7,8 indicated that transverse sinus stenoses resulted in decreased SSS outflow and probable redistribution of venous outflow via smaller collaterals. Also, increased arterial inflow was observed in at least a subset of patients with IIH, suggesting that impaired autoregulation might play a role in the etiology of IIH.7,8 In our study, we wanted to re-evaluate those findings, and in particular, we wanted to find out if and how intracranial flow is influenced by normalization of intracranial pressure via LP.
Our findings indicate that total arterial inflow in patients with IIH is not significantly different from that in healthy controls. The number of patients in our study who did not show distinct sinus narrowing was too small (n = 3) to allow subgroup analysis. Therefore, we cannot confirm that impaired autoregulation resulting in hyperemia plays a role in this subset of patients with IIH, as suggested by Bateman.7,8 Most interesting, compared with us and most authors, Bateman had a much higher percentage of patients with IIH who did not show sinus narrowing (50% and 58%, respectively7,8), which might have influenced his findings.
The largest intracranial sinus, the SSS, showed reduced flow compared with that in controls, possibly related to stenosis-induced outflow impairment via transverse sinuses, but this did not reach statistical significance (P = .08), compared with reports elsewhere.7,8 A reduced SSS outflow could be explained by a compensatory increased venous outflow through other venous outflow pathways. This implies that there is compensatory increased venous outflow through other venous outflow pathways that might have a higher compliance for changes in pressure. These alternative drainage pathways, however, could not be directly assessed because they are too small to be visualized with the MR imaging technique used. These small collateral venous vessels might be sensitive to pressure changes after LP, resulting in increased venous collateral outflow. This could explain why we found a decrease in flow in the IJV after LP but no change in the relatively reduced flow in the SSS.
In the literature, a correlation between elevated intracranial pressure and narrowing of the transverse sinuses has been described,13,15 but resolution of sinus narrowing following the decrease of intracranial pressure by LP has been reported in only a minority of patients.13,24,25 Sinus narrowing normalized in only 2 of our patients following LP though, indicating fixed stenoses in most patients, possibly due to chronic dural sinus wall changes.
It was reported that the PI in the dural sinuses of patients with IIH is lower than in healthy controls, possibly reflecting the increase in dural sinus venous pressure that has been observed by different groups using invasive methods.21 In our patient cohort, lower values of PI in the SSS of patients were found compared with controls. We found a significant negative correlation of the PI with the degree of CSF pressure, supporting the above-mentioned assumptions.
It was reported that venous pressure and flow in the SSS normalized in patients with IIH treated with transverse sinus stents. This outcome, however, does not necessarily seem to be the case in patients treated with LP. We found that SSS flow did not change and RI and PI further decreased after LP. This finding indicates that measuring RI and PI allows visualizing changes in flow dynamics after therapy that have not been seen otherwise. RI and PI could therefore serve as diagnostic markers for a successful reduction in CSF pressure in treated patients. Figure 2 shows an example of a patient having distinct changes in PI and RI in the SSS after LP.26
In this study, we only evaluated the short-term effects of reducing CSF pressure by a single LP. Follow-up studies regarding the long-term effects of a single LP (or multiple LPs), medical treatment, or dural sinus stent placement might also give added insight into pathophysiologic changes in these patients.
In this study, the correlation of intracranial pressure with arterial and venous flow parameters in patients with IIH was measured. In addition to blood flow properties, other parameters might play a role in the assessment of IIH. For a deeper understanding of this complex disease, multifactorial analysis of the whole intracranial system, including CSF flow and pulsatility and elastographic measurements of the brain tissue itself may be helpful.27,28 These additional variables could potentially be used for a better understanding of the pathomechanism of IIH.
Conclusions
Our results indicate changes in several aspects of intracranial blood flow in patients with diagnosed IIH before and shortly after CSF diversion and patients with IIH compared with controls. Venous flow measurements and calculation of resistance and pulsatility indices in combination with dural sinus imaging might provide parameters to distinguish different patient cohorts, help in therapeutic decision-making (eg, stent placement), and monitor effects of CSF-lowering therapies. However, using flow measurements alone for the diagnosis of IIH and evaluating therapeutic strategies might not be sufficient. Therefore, a larger number of patients need to be studied.
Footnotes
Disclosures: Nils Gerd Margraf—UNRELATED: Payment for Lectures Including Service on Speakers Bureaus: Merz Pharmaceuticals and Bayer.
References
- Received April 18, 2017.
- Accepted after revision December 29, 2017.
- © 2018 by American Journal of Neuroradiology