Abstract
SUMMARY: When the first suspected cases of neurologic disorders associated with the Zika virus were noticed in Brazil in late 2015, several studies had been conducted to understand the pathophysiology of the disease and its associated complications. In addition to its well-established association with microcephaly in neonates, the Zika virus infection has also been suggested to trigger other severe neurologic complications in adults, such as Guillain-Barré syndrome, radiculomyelitis, and meningoencephalitis. Hence, the Zika virus should be deemed a global threat that can cause devastating neurologic complications among individuals in all age ranges. The aim of this review was to further describe neuroimaging findings of Zika virus infection and associated neurologic complications found in adults.
ABBREVIATIONS:
- ADEM
- acute disseminated encephalomyelitis
- DENV
- dengue fever
- ELISA
- enzyme-linked immunosorbent assay
- GBS
- Guillain-Barré syndrome
- IgM
- immunoglobulin M
- PRNT
- plaque-reduction neutralization test
- TM
- transverse myelitis
- ZIKV
- Zika virus
The Zika virus (ZIKV) is an arthropod-borne virus (arbovirus) mainly transmitted by 2 species of mosquitoes, Aedes aegypti and Aedes albopictus. The virus was first identified in 1947 in Rhesus monkeys living in the Zika forest in Uganda. During the following decades, cases of human infection were subsequently reported in East and West Africa, Southeast Asia, and, finally, the Americas.1 Other modes of transmission of the virus have been described, such as sexual intercourse, blood transfusion, and vertical (mother-to-child) transmission. No other arboviruses are thought to be transmitted sexually, and the transmission can occur before, during, or after the onset of symptoms and from asymptomatic patients.2,3
The first major reported outbreaks of ZIKV infection were described in French Polynesia in 2013 and 2015. At that time, some neurologic disabilities in infants of infected pregnant women were observed; however, the maternal-fetal transmission was not suspected at that time. Later, further evidence regarding the transmission of the virus was reported in addition to its possible connection to other neurologic complications in adults.1,3
It has been speculated that 500,000–1.5 million people in Brazil have been infected with the ZIKV since the outbreak started.4 French Polynesian visitors are believed to have brought ZIKV to Brazil during the 2014 Fédération Internationale de Football Association World Cup.1 In Brazil, the first case of autochthonous transmission of ZIKV infection was reported in April 2015.5 By the end of 2016, across 1840 municipalities in Brazil, 161,241 cases of probable ZIKV infection were identified, 64,311 of which have been confirmed.5 In the first months of the outbreak, the diagnosis of ZIKV was challenging because its most commonly reported symptoms closely resemble those of other endemic, viral, and exanthematous diseases such as dengue fever (DENV) and chikungunya. Several reports of severe neurologic complications likely to be associated with ZIKV infection have been published during the past 3 years, culminating on February 1, 2016, with a declaration from the World Health Organization of a public health emergency of international concern.6
In addition to its association with microcephaly, the ZIKV infection is also related to other neurologic complications mostly found in adults. Some authors have described a concomitant increase in the incidence of both Guillain-Barré syndrome (GBS) and ZIKV infection.7⇓–9 Association with other neurologic complications in adults has also been suggested, including meningoencephalitis,10 transverse myelitis (TM),11 and ophthalmologic diseases.12
MR imaging plays a key role in assessing the CNS and is, therefore, recommended for the diagnosis and follow-up of patients infected by the ZIKV. MR imaging involves no ionizing radiation and has the advantage of greater tissue differentiation, allowing a better evaluation of the CNS and providing useful data for eventual differential diagnoses.
The aim of this study was to describe the MR imaging findings of neurologic disorders in adult patients with ZIKV infection.
Vectors, Transmission, and Laboratory Diagnosis
The major vector of ZIKV worldwide is the mosquito Aedes aegypti, followed by Aedes albopictus. However, due to widespread distribution, the virus has been recently isolated from other mosquitoes, including the Aedes furcifer, Aedes vittatus, Aedes taylori, and Aedes hirsutus. The ZIKV is primarily transmitted between individuals through infected mosquito bites, establishing a transmission cycle between humans and mosquitoes. The transmission sources of ZIKV described so far are mosquito bites and human-to-human transmission (via body fluids).
It is extremely difficult to differentiate solely on the basis of clinical grounds the acute infections caused by ZIKV, DENV, and chikungunya virus. All of them might present in most patients with an acute exanthematous fever, usually associated with a maculopapular skin rash, conjunctivitis, and a painful syndrome, normally comprising headache, myalgia, and arthralgia, with subtle differences in the presentation.7 The rash might be more prominent with ZIKV, whereas DENV leads to more pronounced headaches and generalized pain, with chikungunya virus frequently causing incapacitating arthralgia and/or arthritis.7 Thus, laboratory diagnosis is essential during an outbreak of arbovirosis, even more so in places with endemic cases.
Since the ZIKV infection has become a global health problem, the World Health Organization has published several algorithms for an accurate diagnosis of the disease.13 The criterion standard for laboratory diagnosis of ZIKV infection relies mostly on the demonstration of the virus in body fluids by real-time reverse-transcription polymerase chain reaction analysis to detect the viral RNA; however, its positivity is very transient, usually persisting no longer than 5–7 days in the blood and CSF.14 Some studies have suggested a prolonged positivity in urine8 and semen15 for a few weeks to months.
The problem is that some of the neurologic manifestations in adults are considered postinfectious autoimmune phenomena (such as GBS, TM, and acute disseminated encephalomyelitis [ADEM]), and by the time of the development of clinical symptoms, viral RNA might be already undetectable. ZIKV immunoglobulin M (IgM) antibody capture enzyme-linked immunosorbent assay (ELISA) might remain positive in the blood and CSF samples for a longer period,13,14 but its use is further complicated in regions with endemic DENV because cross-reactivity is known to occur.13 The plaque-reduction neutralization test (PRNT) can be performed to help differentiate anti-ZIKV antibodies from cross-reacting antibodies, but with a low specificity, excessive cost, and performance only in specialized, experienced laboratories because no commercial kit is available.16 Consequently, since December 2016, the Centers for Disease Control and Prevention no longer recommend the use of the PRNT in areas with endemic presence of other flaviviruses, owing to its low accuracy.14
In an effort to improve diagnostic accuracy, a Brazilian study has shown that cross-reactivity might be overcome by using a ZIKV IgM antibody capture ELISA of both CSF and serum in cases of ZIKV-associated neurologic complications.17 This finding is based on the fact that the IgM pentamer is considered too large to cross the blood-brain barrier and its positivity in the CSF suggests recent intrathecal antibody synthesis and direct CNS penetration of ZIKV.18,19 As suggested by this study, in areas with endemic DENV infection, a serum dengue IgM for serotypes 1–4 should be performed to check for cross-reactivity.17 If both the serum ZIKV and DENV IgM have positive findings, then CSF IgM for both viruses is tested; the results are considered diagnostic if they are positive for ZIKV alone.17 Another recent study from Brazil using CSF and blood samples from neonates with ZIKV-associated microcephaly has further supported these findings, showing a 100% correlation between positive CSF ZIKV serologies and PRNT.20
Neurologic Disorders Associated with Zika Virus Infection in Adults
Guillain-Barré Syndrome.
Guillain-Barré syndrome is an acute, immune-mediated polyneuropathy affecting mainly the peripheral nerves and often following a prior infection. Several reports have described a probable, temporal association between the exponential increase in the number of GBS cases and ZIKV infection in areas of recent ZIKV outbreaks.5,8,17,21 Other arboviruses, especially the dengue and chikungunya viruses, have also been associated with an increased incidence of GBS in previous studies.
Parra et al8 described a series of patients with GBS and positive reverse-transcription polymerase chain reaction for ZIKV in Colombia and showed that most patients presented with an electrophysiologic pattern of acute inflammatory demyelinating polyneuropathy, which was similar to the pattern observed in a prospective cohort in Brazil.17 Conversely, in a case-control study conducted in the French Polynesia, the acute motor axonal neuropathy subtype was the most prevalent presentation.21 Some experts believe that the diverging findings might be explained by differences in performing electrophysiologic testing, rather than some major clinical phenomena.22
In some cases of ZIKV-related GBS, the temporal profile of neurologic symptoms is different from that observed in classic, postinfectious GBS. According to a report, neurologic symptoms are present in almost half of the cases during or immediately after the development of the clinical viral syndrome associated with ZIKV infection.8 Recently, cases of ZIKV-associated acute transient polyneuritis have been reported in Brazilian patients.23 Also, a case of chronic inflammatory demyelinating polyneuropathy has been reported.24 Nevertheless, GBS secondary to ZIKV seems to present with a similar clinical outcome and prognosis compared with more classic triggers for this polyneuropathy, with a fairly similar response to traditional therapies, such as intravenous immunoglobulin.7,8,17,21,25
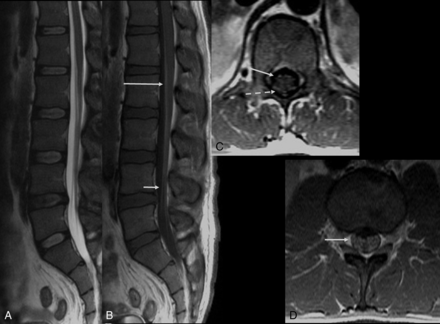
MR imaging is mainly performed to rule out other clinical conditions presenting with acute flaccid paralysis. The findings on MR imaging are like those described in classic GBS, and nerve root enhancement of the cauda equina is often seen in postgadolinium T1-weighted imaging sequences. The involvement of the posterior nerve roots is commonly observed. However, in several cases, diffuse nerve root enhancement may also be observed (Fig 1). Spine MR imaging may also reveal bilateral abnormalities in the lumbar spinal ganglia, characterized by increased signal intensity in T2-weighted images and contrast enhancement. In addition, postcontrast enhancement of cranial nerves may also be noticeable, particularly in the facial and trigeminal nerves (Fig 2).
Guillain-Barré syndrome in a patient with Zika virus infection. Spine MR imaging of a 35-year-old man with progressive ascending paralysis. The conus medullaris and cauda equina nerve roots appear normal on the sagittal T2-weighted image (A). Postcontrast enhancement is noted in the conus medullaris (long arrow) and cauda equina nerve roots (short arrow) following the injection of a gadolinium-based contrast agent in the sagittal T1-weighted image (B). Postgadolinium axial T1-weighted image demonstrates enhancement in both anterior (solid arrow) and posterior (dashed arrow) nerve roots (C) as well as in the nerve roots of the cauda equina (D, arrow).
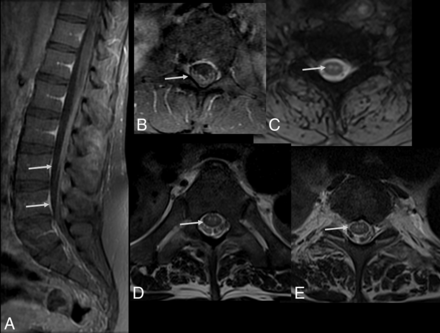
A 30-year-old woman with Zika virus infection with Guillain-Barré syndrome. Postcontrast sagittal T1-weighted image demonstrates enhancement in the cauda equina nerve roots (long arrow) and lumbar spine ganglia bilaterally (short arrows) following injection with a gadolinium-based contrast agent (A). Axial postcontrast, fat-suppressed T1-weighted MR imaging shows gadolinium enhancement of the facial nerves (B, arrows). Axial postcontrast, fat-suppressed T1-weighted MR imaging shows gadolinium enhancement of the trigeminal nerves (C, arrows).
Myelitis.
Despite the current absence of definitive evidence of an association between myelitis and ZIKV infection, a few reports have suggested that this association is likely to occur. Flaviviruses are neurotropic viruses, and DENV, Japanese encephalitis virus, and West Nile virus infections are often related to the occurrence of extensive transverse and longitudinal myelitis.26 Clinically, patients with these infections often present with fast-developing acute flaccid paralysis.
Spinal cord involvement in ZIKV can vary, and previous reports have described different forms of involvement.11,17,27,28 Clinical symptoms are diverse and depend on the location and extension of the lesion. We can speculate that ZIKV might also specifically affect the anterior horns of the spinal cord (Fig 3), leading to a motor neuron syndrome, similar to the West Nile virus.26 Other forms of spinal involvement have been described, such as those present in acute transverse myelitis, which usually involves the spinal cord in >3 segments lengthwise and more than two-thirds of its surface area.17,28
Spine MR imaging of a 35-year-old man with Zika virus infection and Guillain-Barré syndrome presenting with progressive ascending paralysis that evolved to respiratory distress and decreased level of consciousness. The patient had skin rashes preceded by flulike symptoms 1 week before the development of neurologic symptoms. Postcontrast enhancement is seen in the cauda equina nerve roots (arrows) on sagittal (A) and axial (B) T1-weighted spine images following gadolinium-based contrast agent injection. Axial gradient-echo T2-weighted (C) and FSE T2-weighted (D and E) spine images reveal hyperintensity (arrow) in the anterior horns of the cervical (C) and thoracic spinal cord (D).
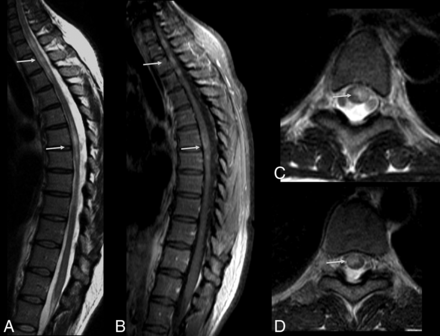
Myelitis can also be identified as diffuse lesions with varying sizes that may affect any portion of the spinal cord. Contrast enhancement may be seen in acute lesions. The spinal cord may be enlarged (Fig 4) due to edema.11,27
Acute myelitis in a patient with Zika virus infection. Spine MR imaging of a 38-year-old woman with unsteadiness and weakness in the lower limbs. Hyperintense, ill-defined lesions are seen in sagittal (A, arrows) and axial (C and D, arrows) T2-weighted images of the cervicothoracic and middle thoracic spinal cord, causing mild expansion of the cord (A). The lesions demonstrate contrast enhancement in the postgadolinium sagittal T1-weighted image (B, arrows).
Meningoencephalitis.
Meningoencephalitis is a well-known and common presentation of Japanese encephalitis29 and West Nile virus infections,26 but it is, surprisingly, a relatively rare complication involving other flaviviruses, including the ZIKV.30,31 The patients affected with meningoencephalitis present clinically with progressive somnolence, seizures, and focal deficits, and in rare cases, their condition may evolve into deep coma or brain death. As demonstrated in previous studies, cases of viral encephalitis (irrespective of the causing agent) may show no abnormalities on imaging examinations. Among a few available reports of ZIKV infection, no specific neuroimaging pattern has been found suggesting the occurrence of this condition.
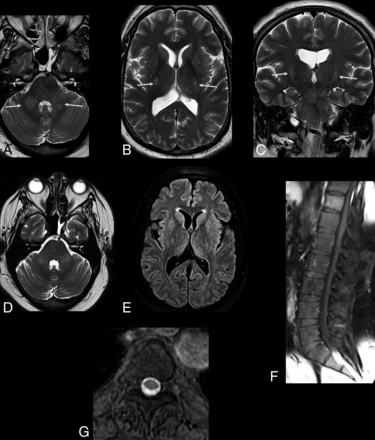
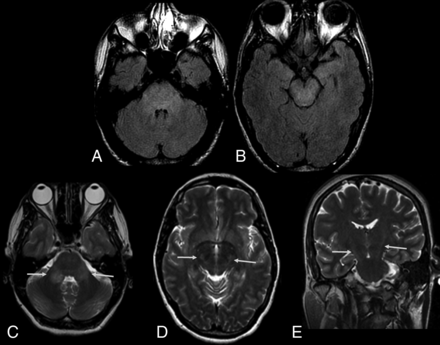
Previous reports have revealed the presence of asymmetric subcortical hyperintense lesions in meningoencephalitis, which may show restricted diffusion.28 Japanese encephalitis and West Nile viruses more commonly affect the deep gray matter, brain stem, and cerebellum; in contrast, a recent report has shown the involvement of the cortical and subcortical junctions, particularly the cingulate and superior frontal gyri.28 Cortical lesions in other infections by flaviviruses have also been demonstrated but are less frequent. In another study, diffuse and confluent lesions in the basal ganglia, thalamus, and white matter have been related to ZIKV infection–related encephalitis.31 Some patients may also present with bilaterally increased signal intensity in the middle cerebellar peduncles and corticospinal tracts (Fig 5). Other patients have been shown to present with brain stem involvement characterizing rhombencephalitis (Figs 6 and 7).
The same patient as in Fig 3. Zika virus-related Guillain-Barré syndrome associated with brain stem encephalitis and myelitis (encephaloradiculomyelitis). Brain MR imaging of a 35-year-old man positive for Zika infection with Guillain-Barré syndrome who presented with progressive ascending paralysis evolving to respiratory distress and decreased level of consciousness. The patient had skin rashes preceded by flulike symptoms 1 week before the development of neurologic symptoms. Axial and coronal T2-weighted brain images show bilateral hyperintensity (arrows) in the middle cerebellar peduncles (A) and corticospinal tracts bilaterally (B and C). Brain and spine MR imaging performed 2 months after treatment demonstrate improvement of the middle cerebellar peduncle and corticospinal tract hyperintensity (D and E). An absence of contrast enhancement is seen in the conus medullaris and cauda equina nerve roots in the postcontrast sagittal fat-suppressed T1-weighted image (F). Axial T2-weighted spine image reveals improvement of the hyperintensity in the anterior horns of the thoracic spinal cord (G).
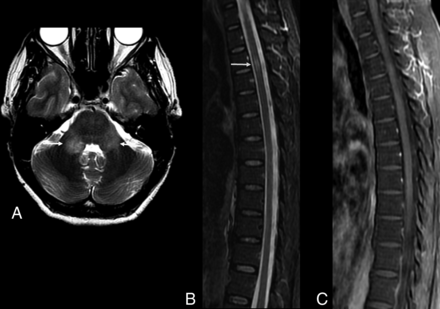
Zika virus–related brain stem encephalitis in a 40-year-old man. Axial fluid-attenuated inversion recovery image shows diffuse hyperintensity in the brain stem, especially in the pons and cerebral peduncle (A and B). Axial T2-weighted images show bilateral hyperintensity in the middle cerebellar peduncles (C, arrows). Hyperintensity is also seen in the corticospinal tracts bilaterally in axial (D) and coronal (E) T2-weighted images. These lesions showed no enhancement on postcontrast T1-weighted images (not shown).
Axial T2-weighted images of a 52-year-old man with Zika virus–related brain stem encephalitis and myelitis. Hyperintense lesions are seen in the upper portion of the lateral columns of the cervical spine (A, arrow) and anterior portion of the medulla bilaterally (B, arrow). The brain stem is diffusively involved (C–G), as are the middle cerebellar peduncles (C) and the bilateral corticospinal tracts (G, arrows).
Frequently, evidence of different forms of presentation may coexist in the same patient, including GBS, myelitis, and meningoencephalitis. In a prospective study conducted in Brazil, cases of concomitant central and peripheral nervous system involvement were observed in some adults with acute ZIKV infection.17
Acute Disseminated Encephalomyelitis.
Acute disseminated encephalomyelitis is an immune-mediated inflammatory demyelinating disorder targeting mostly the white matter of the brain and, less frequently, the gray matter and the spinal cord. It is characterized by an acute or subacute encephalopathy with multiple neurologic deficits and is typically monophasic and self-limiting. Although infrequent, recurrent and multiphasic forms may also occur. Clinical symptom onset generally develops within 3 weeks after a viral infection or vaccination. However, in some cases, it is not possible to prove such a relationship. The diagnosis is based on clinical and imaging findings, as well as on excluding other conditions that can mimic ADEM. Although it can occur at any age, it is more common in children and young adults.
Similar to observations of other viral infections, ZIKV infection may also be related to ADEM.32,33 The imaging findings described are like those observed in other related causes of ADEM, such as multifocal and asymmetric lesions, affecting the brain and the spinal cord. The clinical course of ADEM-ZIKV cases was very similar to that of the most typical ADEM cases, characterized as a self-limited and monophasic course, appearing weeks after the viral infection.
MR imaging findings reveal multiple ill-defined, asymmetric, hyperintense lesions on T2-weighted and FLAIR images (Fig 8), which demonstrate contrast enhancement in T1-weighted postgadolinium images.28 Figure 9 shows follow-up MR imaging performed 2 weeks after the infection.
Brain and spine MR imaging of a 48-year-old woman with Zika virus infection and encephalitis and myelitis. Axial T2-weighted image of the brain shows hyperintensity of the middle cerebellar peduncles bilaterally (A, arrows). Short tau inversion recovery sagittal image (B) demonstrates a hyperintense lesion in the upper portion of the anterior spine without enhancement in the sagittal postcontrast T1-weighted image (C). Follow-up MR imaging was performed 2 weeks later.
The same patient as in Fig 8. Follow-up scans (2 weeks after infection) of the brain and spine MR imaging of a 48-year-old woman with Zika virus infection and encephalitis and myelitis. Improvement of the cerebellar lesions is seen (A, arrows). However, the anterior spinal cord is enlarged (B, arrow) and shows contrast enhancement (C, arrow).
Differential Diagnoses
Some MR imaging findings in ZIKV infection have an appearance like that of other flavivirus infections. The West Nile virus may also present with abnormalities resembling the ones described in ZIKV infections. Previous reports have demonstrated increased signal intensity in both the middle cerebellar peduncle and anterior horns of the spinal cord, as well as contrast enhancement of the lumbosacral nerve roots.26 These imaging features are identical to those observed in some cases of ZIKV infection.17,26 The same pattern may be observed in patients infected with the Japanese encephalitis virus.29 On the other hand, in this particular presentation of patients infected with ZIKV, a slight hyperintensity in bilateral cortical spinal tracts may also be seen (Figs 5⇑–7). Thus, the differential diagnosis of the entities presenting with the imaging findings described above may only be established with further laboratory analysis.
Nerve root enhancement seems to be the most common imaging finding related to ZIKV infection, while it has also been reported that involvement of the lumbosacral nerve roots is mostly related to GBS.8,17 In our opinion, the posterior nerve roots are equally involved in ZIKV infection, as are the anterior nerve roots. However, the anterior nerve roots are preferentially involved in GBS. In endemic areas, the identification of diffuse involvement of the cauda equina may be used to suggest the diagnosis of ZIKV.
Conclusions
In addition to the extensive congenital abnormalities previously described, ZIKV may be associated with a substantially increased incidence of a broad spectrum of life-threatening neurologic syndromes in adults. Unfortunately, no specific neuroimaging finding can be related to this viral infection. In addition, some observed abnormalities are very similar to those seen in other flavivirus infections. However, in combination with clinical information, neuroimaging can be used to suggest the possibility of ZIKV infection, especially in endemic areas. Thus, the MR imaging findings of these neurologic syndromes, in association with serum and CSF analysis as well as molecular and serologic testing, may play a significant role in the diagnosis of ZIKV infection.
Footnotes
This work was supported by the Conselho Nacional de Desenvolvimento e Pesquisa and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro.
Indicates open access to non-subscribers at www.ajnr.org
References
- © 2018 by American Journal of Neuroradiology