Abstract
BACKGROUND AND PURPOSE: MR imaging with sedation is commonly used to detect intracranial traumatic pathology in the pediatric population. Our purpose was to compare nonsedated ultrafast MR imaging, noncontrast head CT, and standard MR imaging for the detection of intracranial trauma in patients with potential abusive head trauma.
MATERIALS AND METHODS: A prospective study was performed in 24 pediatric patients who were evaluated for potential abusive head trauma. All patients received noncontrast head CT, ultrafast brain MR imaging without sedation, and standard MR imaging with general anesthesia or an immobilizer, sequentially. Two pediatric neuroradiologists independently reviewed each technique blinded to other modalities for intracranial trauma. We performed interreader agreement and consensus interpretation for standard MR imaging as the criterion standard. Diagnostic accuracy was calculated for ultrafast MR imaging, noncontrast head CT, and combined ultrafast MR imaging and noncontrast head CT.
RESULTS: Interreader agreement was moderate for ultrafast MR imaging (κ = 0.42), substantial for noncontrast head CT (κ = 0.63), and nearly perfect for standard MR imaging (κ = 0.86). Forty-two percent of patients had discrepancies between ultrafast MR imaging and standard MR imaging, which included detection of subarachnoid hemorrhage and subdural hemorrhage. Sensitivity, specificity, and positive and negative predictive values were obtained for any traumatic pathology for each examination: ultrafast MR imaging (50%, 100%, 100%, 31%), noncontrast head CT (25%, 100%, 100%, 21%), and a combination of ultrafast MR imaging and noncontrast head CT (60%, 100%, 100%, 33%). Ultrafast MR imaging was more sensitive than noncontrast head CT for the detection of intraparenchymal hemorrhage (P = .03), and the combination of ultrafast MR imaging and noncontrast head CT was more sensitive than noncontrast head CT alone for intracranial trauma (P = .02).
CONCLUSIONS: In abusive head trauma, ultrafast MR imaging, even combined with noncontrast head CT, demonstrated low sensitivity compared with standard MR imaging for intracranial traumatic pathology, which may limit its utility in this patient population.
ABBREVIATIONS:
- AHT
- abusive head trauma
- nHCT
- noncontrast head CT
- stMRI
- standard MR imaging
- ufMRI
- ultrafast MR imaging
The incidence of abusive head trauma (AHT) in the United States from 2000 to 2009 was 39.8 per 100,000 children younger than 1 year of age and 6.8 per 100,000 children 1 year of age.1 The outcomes of patients with AHT are worse than those of children with accidental traumatic brain injury, including higher rates of mortality and permanent disability from neurologic impairment.2⇓⇓–5 The diagnosis of AHT is frequently not recognized when affected patients initially present to a physician, and up to 28% of children with a missed AHT diagnosis may be re-injured, leading to permanent neurologic damage or even death.6 Because neuroimaging plays a central role in AHT, continued improvement in neuroimaging is necessary.
Common neuroimaging findings of AHT include intracranial hemorrhage, ischemia, axonal injury, and skull fracture, with advantages and disadvantages for both CT and MR imaging for the detection of AHT.7 A noncontrast head CT (nHCT) is usually the initial imaging study in suspected AHT due to its high sensitivity for the detection of acute hemorrhage and fracture and the high level of accessibility from the emergency department, and it can be performed quickly and safely without the need for special monitoring equipment.8,9 The disadvantages of CT include ionizing radiation, particularly in children, and the reduced sensitivity in detecting microhemorrhages, axonal injury, and acute ischemia compared with MR imaging.10
MR imaging is frequently performed in AHT and adds additional information in 25% of all children with abnormal findings on the initial CT scan.11 Brain MR imaging can also be useful for identifying bridging vein thrombosis, differentiating subdural fluid collections from enlarged subarachnoid spaces, characterizing the signal of subdural blood, and demonstrating membrane formation within subdural collections.12⇓⇓⇓–16 Brain MR imaging findings have correlated with poor outcomes associated with findings on diffusion-weighted imaging and susceptibility-weighted imaging in AHT; however, disadvantages of MR imaging continue to include the need for sedation in children and compatible monitoring equipment.17⇓⇓⇓⇓–22 Although there is greater accessibility of CT compared with MR imaging, the availability of MR imaging is relatively high and imaging techniques that allow neuroimaging in patients with potential AHT without sedation would be valuable, particularly given the potential adverse effects of sedation on the developing brain.23,24
A potential solution for diagnostic-quality brain MR imaging without sedation in AHT is the use of ultrafast MR imaging (ufMRI) sequences, also termed “fast MR imaging,” “quick MR imaging,” or “rapid MR imaging.” Ultrafast MR imaging uses pulse sequences that rapidly acquire images, potentially reducing motion artifacts and the need for sedation. ufMRI has been most commonly used in pediatric neuroradiology for the evaluation of intracranial shunts in children with hydrocephalus, and most of the reported ufMRI brain protocols include only multiplanar T2-weighted HASTE sequences.25⇓⇓⇓⇓⇓⇓⇓⇓–34 Consequently, previously reported limitations of ufMRI in detecting intracranial hemorrhage is primarily due to the lack of blood sensitive sequences.35
Recently, an ufMRI protocol incorporating sequences in addition to T2 sequences has been reported in pediatric patients with trauma.36 This study did not compare findings with those of a standard MR imaging (stMRI) and included a wider age range of pediatric patients, so the value of ufMRI in pediatric abusive head trauma remains uncertain.36 Therefore, the purpose of our study was to evaluate an ufMRI brain protocol performed without sedation for feasibility in terms of scanning time and diagnostic value as well as diagnostic accuracy compared with nHCT and stMRI of the brain for the detection of intracranial traumatic pathology in patients with suspected AHT.
Materials and Methods
Following institutional review board approval, a prospective study was performed from March 2014 through March 2015, evaluating the diagnostic performance of an ufMRI of the brain performed at a tertiary children's hospital in 24 infants who underwent MR imaging for the indication of potential AHT. Infants were eligible for enrollment if they had presented acutely to an emergency department, had undergone an nHCT within the preceding 48 hours either performed at a referring institution or our institution, and were not intubated or sedated for clinical reasons and MR imaging of the head had been requested to further evaluate the patient for potential AHT. The following clinical data were collected for each subject: age, sex, and presentation pediatric Glasgow Coma Scale score. For all patients, an ufMRI brain protocol was performed without sedation and, depending on age, with or without an MRI compatible immobilizer (MedVac Infant Immobilizer; CFI Medical, Fenton, Michigan). At our institution, an immobilizer is routinely used for infants younger than 3 months of age. The ufMRI was immediately followed by an stMRI of the brain with continued use of an immobilizer or with general anesthesia, with a maximum of time interval between the completion of ufMRI and the start of stMRI of 25 minutes in patients requiring sedation. Patients were not excluded if the ufMRI was nondiagnostic but were excluded if stMRI sequences were nondiagnostic.
MR imaging was performed with 1.5T or 3T scanners (Avanto and Verio; Siemens, Erlangen, Germany). The ufMRI and stMRI protocol details are shown in Tables 1 and 2. MR imaging technologists were instructed to repeat an ufMRI sequence only once if there were severe motion artifacts. Technical parameters for nHCT were the following: 100–120 kV(peak); 145–185 mA; and CT dose index, 17.1–29.4 mGy.
Ultrafast MRI brain protocols
stMRI brain protocols
Two board-certified fellowship-trained pediatric neuroradiologists (S.F.K., C.Y.H.) with Certificates of Added Qualification in neuroradiology with 3 and 8 years of experience, respectively, independently reviewed the ufMRIs followed by a review of the stMRIs. Reviewing the ufMRI first without the results of the stMRI allowed a blinded evaluation of the ufMRI. These were reviewed by the same 2 pediatric neuroradiologists at a separate time following a 2-month interval from the MR imaging analysis, to avoid memory bias for nHCT. Axial soft-tissue-algorithm nHCTs at 5-mm section thickness were included for review. Coronal and sagittal reformats were not available in all cases and were not included in the evaluation. The pediatric neuroradiologists were aware that the clinical indication was for evaluation of potential AHT but were otherwise blinded to the final clinical interpretation and additional clinical and radiologic information of the patient, including skeletal survey results.
UfMRIs, nHCTs, and stMRIs were reviewed for subjective diagnostic quality (diagnostic versus nondiagnostic), and specific assessment was recorded for the following: subdural fluid collection (unilateral, bilateral, tentorial, presence of subdural fluid-fluid levels, presence of subdural membrane formation/subdural septation), subarachnoid hemorrhage, epidural hemorrhage, intraventricular hemorrhage, intraparenchymal hemorrhage, cytotoxic edema, nonhemorrhagic vasogenic parenchymal edema, parenchymal lacerations, hydrocephalus, midline shift, herniation (uncal, subfalcine, tonsillar), enlarged subarachnoid spaces, and encephalomalacia.
Subdural fluid collections were defined as fluid collections located under the dura along the convexities, falx, or tentorium. Fluid-fluid levels were defined as a difference in signal intensity or density that had a meniscus/layering pattern. Subdural membrane formation was defined as an identifiable line/band that separated a subdural fluid collection into >1 compartment. Subarachnoid hemorrhage was identified as blood localized within the subarachnoid space including the basal cisterns or that was identified as hyperattenuation on CT and hyperintense signal on FLAIR imaging or hypointense signal on T2*/susceptibility-weighted imaging. Intraparenchymal hemorrhage was defined as intraparenchymal hyperattenuation on CT and focal intra-axial signal abnormality with either low signal on T2-weighted, T2*, or susceptibility-weighted images or high signal intensity on T1-weighted images. Cytotoxic edema was defined as an area demonstrating low attenuation on CT involving gray matter and high signal intensity on DWI with low signal intensity on the corresponding apparent diffusion coefficient map and included diffuse axonal injury and vascular infarct. Nonhemorrhagic vasogenic parenchymal edema was defined as low attenuation on CT sparing the gray matter and abnormal T2 signal hyperintensity without associated intraparenchymal hemorrhage or cytotoxic edema as defined above. Parenchymal lacerations were defined as a parenchymal cleft containing CSF and/or hemorrhage that did not correspond to a normal anatomic structure such as a sulcus. Enlarged subarachnoid spaces were defined as subarachnoid spaces measuring >4 mm in thickness. Encephalomalacia was defined as a focal loss of brain volume involving the cortex identified on any sequence.
On completion of review of the nHCTs, ufMRIs and stMRIs, discrepancies between neuroradiologists were resolved by discussion to establish a consensus interpretation. For the calculation of concordance, an examination was considered “concordant” if all findings were in agreement and “discordant” if there was any disagreement for any of the pathologic categories. κ values < 0 were considered no agreement; 0–0.20, as slight agreement; 0.21–0.40, as fair agreement; 0.41–0.60, as moderate agreement; 0.61–0.80, as substantial agreement; and 0.81–1, as almost perfect agreement.37 Sensitivity, specificity, and positive and negative predictive values for consensus interpretation for ufMRI, nHCT, and ufMRI combined with nHCT, respectively, were calculated compared with consensus stMRI as the criterion standard. The McNemar test was used to assess significance of the discordance rate compared with the criterion standard for each pathologic entity and the changes in sensitivity among ufMRI, nHCT, and combined ufMRI with nHCT. Statistics were performed by using MedCalc statistical software, Version 14.12.0 (MedCalc Software, Mariakerke, Belgium), with P < .05 considered statistically significant.
Results
The median subject age was 4 months (range, 9 days to 31 months), and the male/female ratio was 2:1. The median presentation pediatric Glasgow Coma Scale score was 15 (range, 13–15). As per study protocol, no sedation was performed during ufMRIs of the brain for all 24 patients. stMRI was performed with an immobilizer in 15/24 (63%) patients and with general anesthesia for 9/24 (37%) patients. ufMRI was performed without sedation in all 24 patients, required less than 2 minutes to acquire all of the imaging sequences, and was of diagnostic quality in all patients, while stMRI required general anesthesia in 9 of 24 patients to achieve diagnostic quality and required approximately 15 minutes to acquire all of the imaging sequences. ufMRI sequences and stMRI sequences were considered diagnostic in all patients by both neuroradiologists. Four individual ultrafast MRI sequences were repeated in 3/24 scans compared with a repeat of 11 stMRI sequences in 6/24 scans. All nHCTs were of acceptable diagnostic quality.
A summary of the prevalence of imaging findings identified on stMRI is listed in Table 3. The overall prevalence of patients with an abnormal intracranial trauma finding on stMRI was 83.3%. Binary interreader agreement for complete agreement versus any discrepant finding was moderate for ufMRI (κ = 0.42; 95% CI, 0–0.87), substantial for nHCT (κ = 0.63; 95% CI, 0.30–0.96), and nearly perfect for stMRI (κ = 0.86; 95% CI, 0.60–1). Only 1 patient had an interreader discrepancy on stMRI, which involved the presence of old blood products along the tentorium.
Prevalence of imaging findings per patient on stMRI
Discrepancy rates for individual findings on the consensus interpretation for ufMRI and nHCT compared with stMRI are listed in Table 4. The only significant discrepancy rate by pathology was the detection of intraparenchymal hemorrhage on nHCT compared with stMRI (P = .03). For the total discrepancy rates per examination type, there was significance for consensus ultrafast MRI (P = .004), nHCT (P = .0003), and combined ufMRI and nHCT (P = .01) compared with the criterion standard stMRI.
Discrepancy rates for consensus ufMRI, nHCT, and combined versus stMRI
Discrepancies that consensus ufMRI missed but consensus stMRI detected included the following: 4 patients with subarachnoid hemorrhage, 3 patients with bilateral subdural fluid collections in which 1 collection was not identified, 2 patients with a fluid-fluid level in a subdural collection, and 3 patients with tentorial subdural hemorrhage. ufMRI demonstrated complete agreement between both reviewers; and the stMRI, for the presence of at least 1 subdural collection, intraventricular hemorrhage, parenchymal laceration, enlarged subarachnoid spaces, encephalomalacia, intraparenchymal hemorrhage, herniation or midline shift, and hydrocephalus. There were no abnormal findings described on ultrafast MRI that were normal findings on stMRI. Examples of ufMRI findings compared with stMRI findings are seen in Figs 1⇓–3.
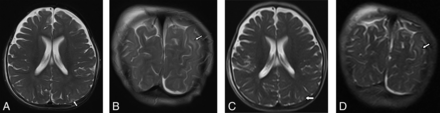
A 4-month-old infant with suspected abusive head trauma found to have bilateral subdural collections identified on coronal T2 TSE (A); however, the right subdural collection was not prospectively identified on ultrafast coronal T2 HASTE (B).
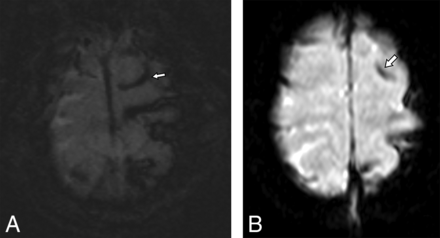
A 31-month-old child with a suspected abusive head trauma with a subdural hematoma (not shown) found to have subarachnoid hemorrhage in the sulci of the left superior frontal and parietal lobes on axial SWI (A), which was prospectively detected by only 1 reviewer on ultrafast axial EPI T2* (B).
A 10-month-old child with suspected abusive head trauma found to have subtle parenchymal edema identified in the left parietal lobe on axial and coronal T2 TSE (A and B), which was not prospectively identified on ultrafast axial or coronal HASTE (C and D).
The diagnostic accuracy of consensus comparisons for each test for detecting any intracranial traumatic pathology with the criterion standard stMRI is listed in Table 5. The differences in the resulting sensitivity of ufMRI versus nHCT and ufMRI versus combined ufMRI with nHCT were not statistically significant (P = .13, P = .48); however, the difference in the sensitivity of combined ufMRI with nHCT versus nHCT alone was statistically significant (P = .02).
Diagnostic performance of consensus ufMRI, nHCT, and combined ufMRI and nHCT compared with stMRIa
Discussion
In this study, we demonstrate that an ufMRI can be reproducibly performed in pediatric patients referred for potential AHT, with subjective diagnostic quality and without sedation. The lack of the need for sedation is considered a primary advantage of ufMRI, and this may allow more institutions to perform brain MRIs on these patients without the requirement of anesthesiology. Indeed, at many institutions that have an MR imaging scanner and even those with 24/7 MR imaging technologist availability, anesthesiology can become a limiting factor for MR imaging in pediatric patients. However, ufMRI may be of little benefit if patients are intubated for clinical reasons because stMRI sequences could be performed without loss of spatial resolution.
Although feasible, ufMRI demonstrated decreased interreader concordance between the reviewers compared with stMRI. Several of the discrepancies could be identified in retrospect on the ufMRI but were likely missed due to thicker sectioning. The most frequent discrepant finding involved detection and localization of subarachnoid hemorrhage, which was better appreciated on SWI than ultrafast axial T2* images, likely due to differences in both spatial resolution and signal intensity. Although many missed findings on ufMRI can be retrospectively appreciated, given that both reviewers have experience in pediatric neuroimaging, the decreased interreader concordance is a limitation of ufMRI compared with stMRI.
Compared with nHCT, ultrafast MRI demonstrated similar discrepancy rates for the detection of subdural and subarachnoid blood, but had significantly improved detection of intraparenchymal hematoma. These findings are likely due to T2* sequences, which detect not only acute blood, which would be bright on nHCT, but also chronic hemosiderin, which would be essentially undetectable on nHCT. Although signal loss on T2* cannot differentiate the chronicity of blood, the detection of blood products not seen on nHCT indicates previous injury and would be helpful when assessing AHT. We did not find differences in the detection of intraparenchymal hemorrhage between ufMRI and stMRI in these patients; however, previous reports have demonstrated a greater sensitivity of SWI compared with gradient recalled-echo for the detection of cerebral microhemorrhage; therefore, we suspect similarly that the ultrafast T2* images will be less sensitive to the detection of cerebral microhemorrhage compared with SWI in a larger cohort.38 The lack of significance for the detection of cytotoxic edema and enlarged subarachnoid spaces between ufMRI and nHCT was not expected because DWI is more sensitive to cytotoxic edema than CT, and T2 HASTE images show the bridging veins within the subarachnoid space more clearly. This unexpected finding may be due to the lower prevalence of these entities in our patient cohort.
Our rationale for combining nHCT and ufMRI is the theoretic algorithm of using both examinations as a potential replacement for stMRI, with nHCT providing greater sensitivity for skull fractures and ufMRI, for parenchymal injury. While this combination does improve sensitivity compared with nHCT alone and raises sensitivity slightly for intracranial pathology compared with ultrafast MRI alone, the overall low sensitivity likely reflects the high sensitivity of SWI on stMRI to small hemorrhages overall, particularly in the subarachnoid space. The decreased sensitivity of ufMRI, nHCT, and the combination of the 2 compared with criterion standard stMRI limits our ability to recommend the use of ufMRI in potential AHT. Institutions that incorporate ufMRI for pediatric patients with trauma should be aware of this potential limitation, and we suggest that if an alternative ufMRI protocol is used, a comparison should be made with an stMRI to assess the accuracy of the ufMRI.
Discrepancies with ufMRI findings may be reduced if these studies are performed more frequently, allowing increased familiarity of the radiologist to the subtleties of ufMRI findings, or they could be avoided by reviewing these studies in consensus. Another possibility would be limiting the use of ufMRI for specific indications such as differentiation of enlarged subarachnoid spaces versus chronic subdural hematomas on nHCT or screening for intracranial trauma in patients with low clinical suspicion for AHT, which can be followed by a later conventional MR imaging if necessary. ufMRI was very accurate for the differentiation of enlarged subarachnoid spaces from subdural collections, a common difficulty with nHCT. If ufMRI is incorporated into clinical use, we recommend a period in which side-by-side analysis with stMRIs is performed before completely replacing stMRI sequences and a low threshold for recommending stMRI.
We could have chosen a broader population to study, particularly any child who came into the emergency department for head trauma, accidental or abusive. However, the included patients in our study are an ideal patient population because of the younger age range, with a higher likelihood of requiring sedation for MR imaging. However, the goal of MR imaging in AHT is not necessarily for acute patient management but for a highly sensitive imaging technique to document intracranial injury in a medicolegal context. One could argue that needing a high level of sensitivity requires neuroimaging with the least amount of error in this patient population and is an ideal challenge to the concept of a fast MR imaging not needing sedation. Because of the need for detail with regard to medicolegal issues, we did not theorize whether the misses on ufMRI without an stMRI would lead to immediate poor patient outcome. Because most of the discrepancies were smaller findings, we would expect a limited effect on immediate patient outcome, not considering the known poor long-term outcomes of a child at risk for abuse. In this regard, ufMRI could play a larger role in screening for intracranial pathology when AHT is unlikely.
Limitations
One limitation of this study is the relatively small sample size. A larger number of patients or a multicenter study may help further the understanding of findings on ufMRI that are reproducibly identified or missed compared with stMRI. Also, the nHCT technique was variable due to inclusion of examinations from referring institutions rather than repeating the nHCT and exposing the patient to additional radiation. Decreasing doses on head CT lessen the signal-to-noise ratio and possibly sensitivity to intracranial pathology. However, our institution is a firm adherent to the Image Gently pledge of the Alliance for Radiation Safety in Pediatric Imaging39 and has a consistently lower dose than our referring institutions. Increasing the radiation dose at the cost of potential increased risk of malignancy seems counterproductive in this sensitive patient population. Finally, the study was performed across both 1.5T and 3T scanners, which have signal-to-noise differences. Because the ultrafast MRI and stMRI examinations were performed on the same magnet, this dichotomy in methodology likely has less effect on our results.
A few of our pathologic categories had zero prevalence in this small patient sample, particularly hydrocephalus, herniation and midline shift, and parenchymal lacerations. This is likely due to the exclusion criterion of intubation, resulting in a neurologically intact patient cohort. Hydrocephalus and mass effect causing herniation and midline shift would not be expected to be missed on ufMRI, given the gross morphologic changes to the brain. However, parenchymal lacerations or subcortical tears are uncommon-but-specific injuries for AHT in very young infants due to immature myelination of the subcortical white matter. Given the small size of these lesions, the sensitivity of ufMRI for this finding is uncertain.
Finally, T1- and T2-weighted FLAIR sequences were conspicuously absent in our ultrafast protocol. These would likely increase both concordance and sensitivity for intracranial pathology. However, these sequences are also sensitive to patient motion due to the length of the acquisition, even with decreasing NEX and matrix size. Optimization of time-versus-image signal and resolution by altering these parameters is a further area of study. Furthermore, motion-correction techniques, such as radial k-space acquisition, may also be beneficial despite the longer time for acquisition.
Conclusions
Diagnostic-quality ufMRI of the brain can be reliably obtained without sedation in patients with potential AHT, and ufMRI requires a very short amount of time to acquire compared with stMRI. However, ufMRI of the brain, as evaluated in our study, demonstrated greater discrepancy between neuroradiologists and had a low sensitivity for intracranial trauma findings, particularly subarachnoid hemorrhage, even when combined with nHCT. These findings limit the use of ufMRI, or a combination of ufMRI and nHCT, as a replacement examination for an stMRI in the imaging work-up of AHT.
Footnotes
Disclosures: Chen Lin—UNRELATED: Grants/Grants Pending: Siemens.* Ralph A. Hicks—UNRELATED: Expert Testimony: University Pediatric Associates Inc,* Comments: Dr Hicks has testified as an expert witness in cases involving suspected child abuse/neglect. Roberta A. Hibbard—UNRELATED: Expert Testimony: Department of Child Services, State of Indiana, Comments: child abuse consultation for attorneys and child protection services*; Grants/Grants Pending: Department of Child Services, Criminal Justice Institute*; Payment for Lectures including Service on Speakers Bureaus: Child First Indiana, Comments: child abuse interviewing; Royalties: Elsevier, Comments: child abuse chapter in dental text; Payment for Development of Educational Presentations: Indiana University School of Medicine, Comments: part of grants.* *Money paid to the institution.
Paper previously presented in part at: Annual Meeting of the American Society of Neuroradiology and the Foundation of the ASNR Symposium, April 25–30, 2015; Chicago, Illinois.
REFERENCES
- Received September 15, 2016.
- Accepted after revision December 5, 2016.
- © 2017 by American Journal of Neuroradiology