Abstract
SUMMARY: Atherosclerosis remains the leading cause of long-term mortality and morbidity worldwide, despite remarkable advancement in its management. Vulnerable atherosclerotic plaques are principally responsible for thromboembolic events in various arterial territories such as carotid, coronary, and lower limb vessels. Carotid plaque ulceration is one of the key features associated with plaque vulnerability and is considered a notable indicator of previous plaque rupture and possible future cerebrovascular events. Multiple imaging modalities have been used to assess the degree of carotid plaque ulceration for diagnostic and research purposes. Early diagnosis and management of carotid artery disease could prevent further cerebrovascular events. In this review, we highlight the merits and limitations of various imaging techniques for identifying plaque ulceration.
ABBREVIATIONS:
- CE-MRA
- contrast-enhanced MRA
- CDUS
- color Doppler ultrasound
- CEUS
- contrast-enhanced ultrasound
- US
- ultrasound
- XRA
- x-ray contrast angiography
Stroke is considered the leading cause of death and long-term disability worldwide.1 Carotid atherosclerosis is one of the major causes of ischemic stroke.2 Morphologic features such as plaque ulceration are strongly correlated with ischemic stroke and coronary events, with hazard ratio ranges from 1.2 to 7.7,3⇓⇓⇓⇓–8 as summarized in Table 1. The hazard ratio is comparable with other high-risk factors such as large lipid core (hazard ratio = 1.75) and intraplaque hemorrhage (hazard ratio = 5.85).9
Summary of the hazard ratios of carotid ulceration for future events risk in different studies
Carotid plaque ulceration or surface irregularity is characterized as an indentation, fissure, or erosion on the luminal surface of a plaque, exposing a portion of the inner plaque to direct contact with the circulating blood.10 Various factors are involved in the pathogenesis of ulceration, including the accumulation of inflammatory cells, proteolytic enzymes released by macrophages, and local hemodynamic factors.11 These factors weaken the fibrotic cap, leading to plaque rupture and leaving behind the ulceration. These ulcerations act as a thromboembolic source, allowing plaque components to be released into the blood. Ulcerated plaques are considered the main foci of cerebral microemboli.12
Plaque ulceration can be visualized grossly following carotid endarterectomy and later by histologic analysis of the specimen. Figure 1 shows histologic images of an ulcerated plaque.13 Early detection of plaque ulceration before an operation is essential because it may assist in preventing further thromboembolic events; therefore, there has been substantial research to evaluate different radiographic techniques in the early identification of plaque ulceration.
Histologic section of an ulcerated plaque by using a hematoxylin-eosin stain showing the ulceration (left). The CD68 stain shows macrophages (middle), and the smooth-muscle actin stain shows a lack of smooth-muscle cells (right). Reprinted with permission from Gillard et al.13 Copyright Cambridge University Press 2007.
Various imaging modalities are used to assess plaque ulceration for diagnostic and research purposes (Table 2 and On-line Table). These include x-ray contrast angiography (XRA), B-mode and Doppler sonography, CTA, and MRA. The purpose of this article was to compare the different clinical imaging modalities in observing carotid ulceration from existing literature and evaluate the diagnostic value of each method.
Summary of details in each imaging modality
X-Ray Contrast Angiography
X-ray contrast angiography, including conventional carotid angiography or DSA, is an established method of assessing carotid artery disease. Conventional angiography involves the acquisition of digital fluoroscopic images in combination with the administration of an iodinated contrast medium. DSA produces the angiography by subtracting the postcontrast images from precontrast images to achieve better visualization of the blood vessels. Previously, XRA was considered a criterion standard for the assessment of carotid artery disease because of its high spatial (50 μm) and temporal resolution (10 ms). It has the ability to depict the stenotic lumen and various plaque characteristics such as surface irregularities or large ulcerations. It has the advantage of visualizing a long segment of the artery at a single time point.
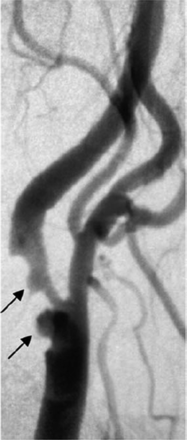
XRA has been widely used in large, randomized clinical trials, such as the North American Symptomatic Carotid Endarterectomy Trial (NASCET),14 the European Carotid Surgery Trial (ECST),15 and the Asymptomatic Carotid Atherosclerosis Study (ACAS).16 A study comparing angiographic surface morphology with detailed histology has concluded that ulceration detected by XRA was associated with plaque rupture, intraplaque hemorrhage, and overall plaque instability.17 An example of plaque ulceration on XRA is shown in Fig 2.
DSA image of 2 large ulcerations (arrows) of a right internal carotid artery. Reprinted with permission from Gillard et al.13 Copyright Cambridge University Press 2007.
However, there are several limitations to the extensive use of XRA, especially in the carotid territory. XRA involves ionizing radiation. It is a high-cost and time-consuming procedure and requires adequate bed rest after the investigation. The invasive nature of this procedure increases the risk of creating emboli, resulting in subsequent cerebrovascular events.16,18,19 In an article based on ACAS, there was a 1.2% risk of persisting neurologic deficits or death following XRA, while the surgical risk was only 1.5%.16 Another article based on NASCET showed that a 0.7% risk of persistent neurologic deficits or death was associated with the angiography.19 XRA is not safe in patients with coagulopathies and bleeding disorders. The accuracy of XRA in detecting ulceration also depends on the degree of stenosis.20 Finally, the rates of false-positives and false-negatives of XRA were high in identifying ulcerations.21 Two possible reasons for its low accuracy in detecting ulceration are that it is operator-dependent and DSA generally acquires only a limited number of projections. These issues result in failures to detect ulceration21,22 and a tendency to underestimate stenosis.23
Based on the above-mentioned reasons, there has been a trend to replace XRA with alternative cost-effective, safe, and less time-consuming carotid imaging modalities, which are discussed below.
Sonography
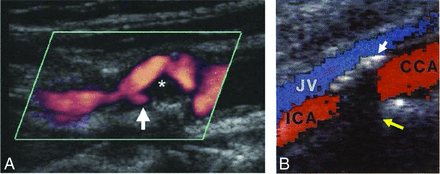
Sonography was introduced as the first platform to visualize the in vivo human vessel and atherosclerosis.24 It helps to classify the plaque texture as either homogeneous (uniform consistency) or heterogeneous (nonuniform consistency).25 Homogeneous plaques present with a uniform echo intensity and show a regular, smooth surface, while heterogeneous plaques show a nonuniform pattern with mixed echo intensities and usually have an irregular/ulcerated surface.25 The plaque surface can be defined as smooth and regular, mildly irregular, or ulcerated in the case of a variation in height between 0.4 and 2 mm on the contour of the plaque.26 An example of ulceration in Doppler sonography is shown in Fig 3A. However, it is difficult to detect plaque ulceration by sonography due to various limitations. First, the overall accuracy of using B-mode sonography against criterion standard techniques (DSA or histopathology) is not high (sensitivity and specificity ranges from 39% to 89% and 72% to 87%, respectively).27⇓–29 Several studies have noted that its accuracy decreases with the increasing degree of stenosis,30,31 and it has even failed to detect ulceration in high-grade stenosis.29 The application of color-flow Doppler-assisted duplex imaging, which combines the B-mode and blood-flow velocity information,32 also shows limitations in providing adequate information to identify plaque ulcerations.31,33 Second, the intrareader reproducibility of both B-mode and Doppler sonography is low (κ ranges from 0.11 to 0.8931,34⇓–36), which is not sufficient for reliable diagnosis. Third, the criteria for carotid ulceration diagnosis are very subjective and may vary from reader to reader or center to center; this variation makes its use difficult for multicenter trials.37
A, Doppler sonography shows an internal carotid artery plaque ulceration (white arrow) The asterisk shows weakly echogenic plaque material, presumably lipid. Reprinted with permission from Gillard et al.13 Copyright Cambridge University Press 2007. B, The calcification in the anterior vessel wall (white arrow) shadows the color Doppler signal and opposite wall structures (yellow arrow). JV indicates jugular vein; CCA, common carotid artery. Adapted from Steinke et al.39
These limitations are mostly due to the native imaging principle. 2D sonography can only obtain a 2D cut plane of the carotid area; this could introduce operator error when the sonography probe is not parallel to the vessel axis or the orientation of the ulceration.38 Also, the presence of calcification reflects the acoustic wave, which can obscure ulceration.38 An example of artifacts due to calcification is shown in Fig 3B.39
The use of microbubble contrast agents has been shown to improve accuracy. A direct comparison of contrast-enhanced ultrasound (CEUS) and color Doppler ultrasound (CDUS) observed that CEUS has superior sensitivity and diagnostic accuracy over CDUS in detecting ulceration.40 Within the same study, CEUS detected more ulceration than CTA, especially small ulcerations, attributed to the higher spatial and temporal resolution achieved in CEUS.40 Further CEUS studies will be required to verify the improved accuracy of this technique. The safety of using CEUS should also be considered, including toxicity, microembolism, and inertial cavitation caused by the microbubbles.41
The recent development of 3D sonography has demonstrated superior ability in detecting ulceration compared with conventional 2D sonography (Fig 4).36,42 3D sonographic images can be obtained by using dedicated 3D probes or by using 2D sonographic probes with the help of positioning sensors and postreconstruction algorithms to combine 2D sections into a 3D volume.43,44 This process improves image quality, provides more information about plaque morphology and echomorphology, and has been used to noninvasively quantify plaque stenosis45 and volume46,47 and examine the regression and progression of plaque ulceration.42 By comparing 3D and 2D sonography in 142 patients, Heliopoulos et al36 showed that 3D methods depicted more ulcerations than the 2D methods (15% versus 8% of plaques) and also had higher interobserver reproducibility (κ = 0.973, standard error = 0.027, versus κ = 0.885, standard error = 0.055). However, this methodology is still under development and requires further validation against accepted criterion standard techniques such as DSA and histopathology.
A, 2D sonography depicts a smooth plaque, arrow shows the stenosis. B, 3D sonography shows an ulceration of the same plaque in another plane, arrow shows an ulcer at site of shear stress. The figure is adapted with permission from Heliopoulos et al.36
CTA
Studies with CTA have demonstrated that plaque ulceration is closely associated with increased lipid volume,48,49 an increased degree of stenosis,38 plaque volume, and decreased calcification proportions.49 Surgical observations have shown good correlation, with CTA having a high sensitivity (94%) and specificity (99%) to detect plaque ulceration.50 Compared with sonography, CTA showed higher sensitivity and specificity to detect ulceration.38
In comparison with DSA, CTA has fewer associated complications,38 while its accuracy in the determination of ulceration still needs more research for validation. One major limitation of CTA in detecting ulceration is the appearance of plaque calcification.51
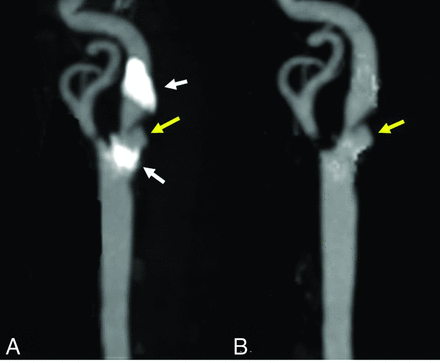
The recent development of dual-source CT, which uses 2 x-ray energies simultaneously to separate high-density calcification and the contrast-enhanced lumen, has shown advantages for evaluating densely calcified carotid stenosis and could be more accurate.52,53 Figure 5 shows that the morphology of ulcerations cannot be visualized clearly by conventional CTA due to calcification, while dual-source CTA software could remove the calcification from the image, making the ulcer clearer.
A, An ulceration (yellow arrow) in a heavily calcified (white arrows) plaque. B, The ulcer is clearer with the calcification removed by dual-energy CTA.
Like XRA, a drawback of CTA is the use of ionizing radiation. In imaging the neck vessels, the radiation dose of CTA is equivalent to or higher compared with that in DSA.54,55 Also, the use of contrast media may be contraindicated in some patients with poor renal function.56
MR Imaging
Noncontrast-Enhanced MRA.
The most common method for MRA is time-of-flight, which relies on the high MR imaging signal from the moving blood within the vessel lumen to create vascular contrast.57 Both 2D (ie, multi-slice58) and 3D (ie, volumetric57,59) TOF have been used for carotid artery imaging. One of the biggest advantages of MRA over DSA and US is that the images can be reformatted into any orientation after the acquisition.
However, one of the well-known limitations of TOF-MRA is that signal saturation and dephasing of the signal could lead to a signal loss from focal areas of complex flow.60 The stenosis measurement accuracy of TOF is dependent on the wash-in efficiency of unsaturated spins within the imaging section/slab. For large ulcerations, the hemodynamic patterns of blood flow are complicated.61 Ulceration detection could therefore be limited if the saturated spins are not replaced by fresh unsaturated blood flow. Also, the orientation of the imaging section/slab is important. TOF techniques are limited to the flow orthogonal or at a certain angle to the imaging sections/slabs. The signal from flowing blood parallel to the imaging sections/slabs can become saturated.59 In addition, the ulceration orientation, location, and shape could also influence the accuracy of measurements with TOF-MRA.61 Spatial resolution would be another limitation of TOF-MRA, especially for very small ulcers.59 In addition, patient motion during relatively long acquisition times is another limitation.59
In recent years, other non-contrast-enhanced MRA techniques have emerged claiming to overcome some of the limitations of TOF-MRA. Arterial spin-labeling–based methods subtract images where fresh flowing blood has been magnetically “labeled” from images without labeling. Such methods have demonstrated the ability to image arteries of the head and neck without signal from static background.62,63 In particular, a hybrid of pseudocontinuous and pulsed arterial spin-labeling with a fast low-angle shot readout has shown similar results in detecting carotid luminal irregularity with contrast-enhanced MRA (CE-MRA) and overcomes some of the limitations of TOF-MRA (Fig 6).63 The inversion recovery–based methods64 and the quiescent interval low-angle shot method65 use in-plane saturation pulses to suppress the background signal, allowing only the nonsaturated inflowing blood to be imaged. Blood-suppression–based methods use the subtraction of images with and without blood-suppression preparation pulses and have also shown good images of arteries and veins.66 Because these methods have only recently been developed, more studies are necessary to validate their accuracy in detecting plaque ulceration.
Luminal irregularity in the internal carotid artery is demonstrated on both a nonenhanced hybrid of pseudocontinuous and pulsed arterial spin-labeling (arrow, A) and CE-MRA (C) images, but it is not seen on the 3D TOF image (dashed arrow, B). hASL indicates hybrid of pseudocontinuous and pulsed ASL. The figure is reproduced with permission from Koktzoglou et al.63
Contrast-Enhanced MRA.
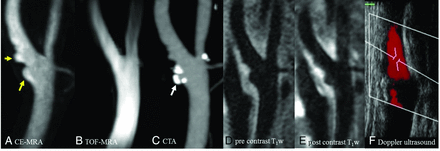
CE-MRA is an MR imaging technique for vascular imaging that exploits the use of an intravenously administered paramagnetic contrast agent (ie, a chelate of gadolinium) to shorten the T1 relaxation time of the blood, providing excellent contrast with the background tissues. Because the images are no longer dependent on the inflow of the blood, CE-MRA produces high-quality images in a short timeframe and may reduce some of the drawbacks associated with TOF-MRA. In 1 study, the prevalence of plaque ulceration was 86% in a symptomatic patient cohort compared with 36% in an asymptomatic patient group, indicating that CE-MRA could be used for detecting ulceration.67 CE-MRA has also been shown to detect more ulcers than TOF-MRA.61 Figure 7A shows an internal carotid artery with several ulcerations demonstrated by CE-MRA; however, all were missed by TOF-MRA (Fig 7B). In addition, CE-MRA has the advantage of depicting ulceration in calcified plaques, which is one of the limitations of standard CTA (Fig 7C). The images were processed by using a dedicated workstation (Advantage Windows 4.6; GE Healthcare, Milwaukee, Wisconsin).
High-resolution MR imaging, CTA, and sonography of the left carotid artery of a 77-year-old man. Ulcerations (yellow arrow) are shown clearly on CE-MRA (A) and pre- and postcontrast black-blood T1-weighted (D and E) images; however, they were missed on TOF-MRA (B). The calcification on CTA (white arrow, C) causes difficulty when observing the ulceration. Doppler sonography (F) shows no ulceration in the internal carotid artery.
Although CE-MRA shows high accuracy in detecting plaque ulceration, it is still a relatively expensive examination. MR imaging is not suitable for patients with contraindications such as implanted devices. In addition, the use of gadolinium-based contrast agents may be contraindicated in patients with severe renal impairment (eg, glomerular filtration rate < 30), which may limit its wider application.
Blood-Suppressed MR Morphologic Imaging.
High-resolution standard MR images are widely used for carotid morphologic imaging; however, the signal from flowing blood in the lumen makes it difficult to identify the vessel wall. Blood suppression is usually achieved through a signal-preparation scheme applied before the imaging sequence. The most commonly used schemes include double or quadruple inversion recovery,68,69 motion-sensitive driven equilibrium,70 and delay alternating with nutation for tailored excitation (DANTE).71 Multicontrast cross-sectional MR imaging with blood could also be used for ulceration detection.72,73 Figure 7D, -E shows an example of carotid ulceration in DANTE-prepared pre- and postcontrast T1-weighted images.
Discussion
Carotid ulceration is now considered a major hallmark in determining the vulnerability of atherosclerotic plaque because it indicates a previous plaque rupture and is a strong predictor of subsequent events. The identification of plaque ulceration may assist in the appropriate management of patients at risk of future ischemic events. We have reviewed the literature regarding the various radiologic techniques used to demonstrate plaque ulceration.
A direct comparison of the sensitivity and specificity of different imaging modalities is difficult because the definition of plaque ulceration varies in different studies. Pathologically, ulceration is defined as an erosion of the single cell–layer intima by microscopic examination74⇓–76 or surface defects more than a certain value (such as 560 μm or 1 mm in diameter and depth) in gross photography.12,28,29,77 In some studies with DSA,17 CTA,49,78 and MRA,67,79 a general definition “the extended lumen into plaque” has been used. In some of the CTA studies, a more specific definition has been described, such as the intimal defect must be larger than 1 mm in width38,48,50 or 2 mm in depth.80
Sonography is limited by its accuracy and reproducibility, especially when the lesion is calcified. The recent development of 3D US and the use of CEUS may help improve the detection of carotid ulceration.
CTA is relatively safe compared with XRA and much faster and cheaper than MR imaging. However, as with XRA, ionization must be considered when using CTA. Optimization of the scanning protocol and the use of new reconstruction techniques81 can help reduce the radiation dose. The application of dual-source CTA may also help to improve the sensitivity and accuracy in detecting ulceration within calcified plaques.
The advantage of MR imaging is that morphologic and functional features of carotid plaque can be obtained within a single examination. These features could help provide a comprehensive assessment of plaque vulnerability. Non-contrast-enhanced MRA techniques have shown comparable efficiency with CE-MRA for detecting ulceration and could be used in patients with contraindications to contrast agents. By improving the resolution and optimizing the acquisition sequence, non-contrast-enhanced MRA techniques may identify smaller ulcerations missed by current MR imaging methods.
Indicates open access to non-subscribers at www.ajnr.org
REFERENCES
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.
- 83.
- 84.
- 85.
- 86.
- 87.
- 88.
- 89.
- © 2017 by American Journal of Neuroradiology