Abstract
BACKGROUND AND PURPOSE: Reaction time was recently recognized as a marker of subtle cognitive and behavioral alterations in the early clinical stages of CADASIL, a monogenic cerebral small-vessel disease. In unselected patients with CADASIL, brain atrophy and lacunes are the main imaging correlates of disease severity, but MR imaging correlates of reaction time in mildly affected patients are unknown. We hypothesized that reaction time is independently associated with the corpus callosum area in the early clinical stages of CADASIL.
MATERIALS AND METHODS: Twenty-six patients with CADASIL without dementia (Mini-Mental State Examination score > 24 and no cognitive symptoms) and without disability (modified Rankin Scale score ≤ 1) were compared with 29 age- and sex-matched controls. Corpus callosum area was determined on 3D-T1 MR imaging sequences with validated methodology. Between-group comparisons were performed with t tests or χ2 tests when appropriate. Relationships between reaction time and corpus callosum area were tested using linear regression modeling.
RESULTS: Reaction time was significantly related to corpus callosum area in patients (estimate = −7.4 × 103, standard error = 3.3 × 103, P = .03) even after adjustment for age, sex, level of education, and scores of depression and apathy (estimate = −12.2 × 103, standard error = 3.8 × 103, P = .005). No significant relationship was observed in controls.
CONCLUSIONS: Corpus callosum area, a simple and robust imaging parameter, appears to be an independent correlate of reaction time at the early clinical stages of CADASIL. Further studies will determine whether corpus callosum area can be used as an outcome in future clinical trials in CADASIL or in more prevalent small-vessel diseases.
ABBREVIATIONS:
- BPF
- brain parenchymal fraction
- CCA
- corpus callosum area
- MB
- microbleeds
- RT
- reaction time
- SE
- standard error
- SVD
- small-vessel disease
Ccerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is a monogenic cerebral small-vessel disease (SVD) caused by mutations of the NOTCH3 gene.1 In unselected patients, brain atrophy and the volume of lacunes are the main MR imaging markers of disease severity.2,3 By contrast, in nondisabled patients without dementia, CADASIL may present without brain atrophy and with few if any lacunes, and MR imaging correlates of disease severity are unknown. Simple and robust MR imaging markers at these early stages would be of interest because future therapeutic trials will likely include the patients with the least severe diseases.
We recently showed that reaction time (RT) is a marker of subtle cognitive and behavioral alterations observed in nondisabled patients without dementia with CADASIL.4 The MR imaging correlates of RT in these patients are undetermined.
Both in healthy aging and in sporadic SVD, the corpus callosum area (CCA) reflects white matter integrity and is related to various estimates of processing speed.5,6 In the present study, we aimed to test whether RT is independently related to CCA in nondisabled patients with CADASIL without dementia.
Materials and Methods
Study Participants
Twenty-six patients with genetically confirmed CADASIL followed within a prospective cohort study in the French Referral Centre for Rare Vascular Diseases of the Eye and the Brain (CERVCO, http://www.cervco.fr) were included. Within our referral center, about 250 patients with CADASIL are evaluated every 18 months with a comprehensive neuropsychological battery by an experienced neuropsychologist (S.R. or A.J.). Patients were invited to participate in the present study if they presented at the most recent evaluation (within 18 months) without global cognitive alterations as confirmed by the patient and his or her relatives; if neuropsychological testing scores were within the expected range according to the patient's age and level of education with a Mini-Mental State Examination score superior to 24 and did not have significant disability (modified Rankin Scale score of 0 or 1). Patients with overt motor deficits were excluded, but most reported minor poststroke residual symptoms such as gait disturbances or focal sensory deficits. Given the fluctuations of subjective cognitive and behavioral symptoms, particularly regarding fatigue and concentration issues, they are not systematically recorded at each visit and the evaluations reported in the present study are only based on objective measures. The present group of 26 patients included 22 patients for whom relationships between RT and cognitive alterations were reported previously4 and 4 additional patients recruited afterward. Twenty-nine age- and sex-matched control subjects were recruited from a local data base of healthy volunteers free of any history of neurologic or psychiatric disorders (except migraine). None of the patients or controls had a history of head trauma or demyelinating disease. An ethics committee validated the study protocol. All patients and controls gave their informed consent for participating in the present study.
Neuropsychological Assessment
All participants underwent a comprehensive neuropsychological evaluation, details of which have been published previously.4 RT was measured with a simple computerized task. Subjects were asked to click a mouse button as soon as a white X appeared on the screen for a maximum duration of 2000 ms. Twenty stimuli were presented sequentially during each trial, and 4 trials were performed in a row. During trials 2 and 3, the visual stimulus was primed by an auditory stimulus presented with a random time interval varying between 100 and 1000 ms. RT was recorded in milliseconds for each subject on the basis of the complete results obtained after 4 trials. We used the median RT based on the 4 trials for analyses. The total duration of this simple task was about 5 minutes. The Mini-Mental State Examination was used as a measure of global cognitive performance,7 and the presence of depression was defined according to the Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Text Revision criteria,8 and the Montgomery-Asberg Depression Rating Scale score was obtained for all subjects.9 Subjects were considered apathetic if they had a Starkstein Apathy Scale score of ≥14 and fulfilled the Starkstein Structured Clinical Interview for Apathy criteria.10
MR Imaging Acquisition and Processing
The original MR imaging protocol included 3D T1-weighted images obtained at 3T with a Tim Trio scanner (Siemens, Erlangen, Germany) equipped with a 12-channel head coil, with a standard sagittal magnetization-prepared rapid acquisition of gradient echo sequence (in-plane resolution, 1 × 1 mm2; section thickness, 1.1 mm; TR, 2300 ms; TE, 2.98 ms; TI, 900 ms; flip angle, 9°; bandwidth, 238 Hz/pixel; and acquisition time, 7 minutes 45 seconds). FLAIR and T2*-weighted sequences were not part of the present MR imaging protocol, but all patients also underwent a standard MR imaging protocol on a 1.5T scanner (Signa; GE Healthcare, Milwaukee, Wisconsin) within 6 months. The FLAIR sequence was used for the segmentation of white matter hyperintensities of presumed vascular origin, and the T2* sequence, for counting the number of microbleeds (MB), using previously validated methodologies.2,11 Brain parenchymal fraction (BPF), defined as the ratio of brain volume to the intracranial cavity volume, and the volume of lacunes were determined from the 3D-T1 sequence in all subjects.12 Masks of lacunes were obtained manually by a trained rater. Hypointense lesions with a signal identical to that of CSF, sharp delineation, and a diameter of >2 mm were selected for this segmentation. Lacunes were distinguished from perivascular spaces by using STandards for ReportIng Vascular changes on nEuroimaging recommendations, including size criterion.11
The 3D-T1 scans were manually registered to the midsagittal plane, with rotation transforms in Multi-image Analysis GUI (Mango; Research Imaging Institute, University of Texas Health Science Center at San Antonio; http://ric.uthscsa.edu/mango). Anterior/posterior commissures and the aqueduct of Sylvius were aligned on the interhemispheric plane. Once re-aligned, scans were processed with manual drawing in Anatomist (www.brainvisa.info) to delineate the corpus callosum on noninterpolated images (Fig 1). This approach is generally considered the reference method.13 Given the strong links between head size and the area of the corpus callosum,14⇓–16 CCA was normalized to the intracranial cavity volume (ICV) while raising the CCA at the power 3/2 to obtain a dimensionless value (CCA3/2/ICV). Intra- and interrater reliability were evaluated in a sample including 10 patients and 10 controls selected randomly from the whole sample of 55 subjects. Intraclass correlation coefficients were 0.94 and 0.92, respectively.
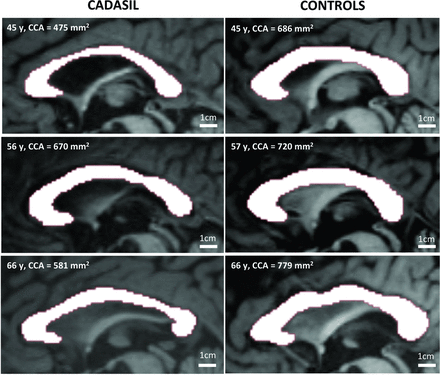
Examples of corpus callosum area delineation in 3 patients with CADASIL and 3 age-matched controls. Reproduction at the same scale of noninterpolated midsagittal T1-weighted images of the corpus callosum in 3 patients (left column) compared with 3 age-matched controls (right column). Corpus callosum atrophy appears obvious in some cases.
To further explore the relationships between CCA and lacunes, we used a template of the corpus callosum from the ICBM-DTI-81 white matter atlas (http://www.loni.usc.edu/ICBM/Downloads/Downloads_DTI-81.shtml),17 which was thereafter projected into the native space of each patient to systematically identify lacunes within the central part of the corpus callosum.
Statistical Analysis
Statistical analyses were performed with R statistical and computing software (http://www.r-project.org). Between-group comparisons were performed with t tests for continuous variables with normal distributions. Wilcoxon tests were used otherwise. For categorical variables and comparisons of proportions, χ2 tests were used. Multiple linear regression modeling was used to test the relationship between CCA and MR imaging markers or between RT and CCA. Analyses were adjusted when necessary for the following variables, based on data from the literature: age, sex, level of education, and scores of depression and apathy. For all analyses, P < .05 (2-tailed) was considered significant.
Results
Characteristics of patients and controls are given in the Table. In line with our previous results,4 patients had significantly longer RTs than controls (288 ± 56 ms versus 253 ± 43 ms, P = .02). Patients also had significantly greater scores of depression (Montgomery-Asberg Depression Rating Scale: 5.9 ± 6.9 versus 1.7 ± 3.0, P = .007) and a significantly lower level of education (11.3 ± 3.7 years versus 13.5 ± 3.5 years, P = .02), but the difference in RT between patients and controls remained significant after adjustment for age, sex, level of education, and scores of depression and apathy (estimate = 29.8, standard error [SE] = 14.8, P = .04).
Characteristics of patients with CADASIL and control subjects
CCA was significantly smaller in patients (estimate = 1.3 × 10−2 ± 3.0 × 10−3 versus 1.6 × 10−2 ± 3.0 × 10−3, P < .001), even after adjustment for age and sex (estimate = 3.3 × 10−3, SE = 7.6 × 10−4, P < .001). By contrast, patients showed no brain atrophy (BPF: in patients, 0.84 ± 0.03, versus controls, 0.84 ± 0.03; P = .77), even after adjustment for age and sex (estimate = −2.5 × 10−3, SE = 7.1 × 10−3, P = .72).
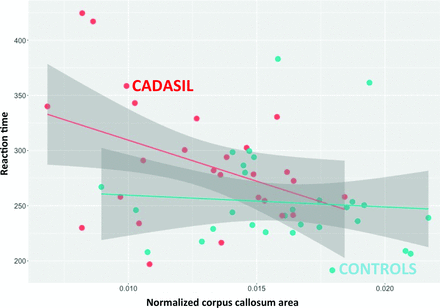
In patients, RT was negatively related to CCA both before (estimate = −7.4 × 103, SE = 3.3 × 103, P = .03) (Fig 2) and after adjustment for age, sex, level of education, and score of depression and apathy (estimate = −1.2 × 104, SE = 3.8 × 103, P = .005). Further adjustment for the number of MB, the volume of white matter hyperintensities, volume of lacunes, and BPF did not alter our results (estimate = −1.4 × 104, SE = 5.3 × 103, P = .02).
The relationship between reaction time and CCA in patients with CADASIL and in controls. The mean reaction time appears significantly and negatively associated with the corpus callosum area in patients (estimate = −7.4 × 103, SE = 3.3 × 103, P = .03), while no association is observed in controls (estimate = −1.1 × 103, SE = 2.6 × 103, P = .69).
In patients, there was no significant association between RT and any of the classic MR imaging markers: number of MB (estimate = −1.05, SE = 2.07, P = .62), volume of white matter hyperintensities (estimate = 1.7 × 10−4, SE = 1.6 × 10−4, P = .30), volume of lacunes (estimate = −3.3 × 10−2, SE = 2.5 × 10−2, P = .20) and BPF (estimate = −4.1 × 102, SE = 4.4 × 102, P = .37).
In controls, there was no significant association between RT and CCA (estimate = −1.1 × 103, SE = 2.7 × 103, P = .69).
In patients, CCA was negatively related to the volume of lacunes (estimate = −3.1, SE = 0.9, P = .002). Results were unchanged when we removed the 3 patients with lacunes within the central part of the corpus callosum (data not shown) from our analysis. Additionally, CCA was positively related to BPF (estimate = 4.6, SE = 1.5, P = .008). By contrast, there was no significant association with the volume of white matter hyperintensities (estimate = −0.4, SE = 7.6, P = .96) or the number of MB (estimate = −1.2, SE = 0.8, P = .12).
Discussion
In the present study, we found that CCA is an independent MR imaging marker of reaction time at the early clinical stages of pure SVD. By contrast, other conventional MR imaging markers were not associated with reaction time. In this sample of patients with CADASIL without disability and without dementia who had no brain atrophy and few if any lacunes, CCA was lower than in age- and sex-matched controls. The effect of the volume of lacunes on CCA was not mediated only by the 3 patients having lacunes on its most central part. This suggests that CCA somehow summarizes the widespread effect of lacunes on its fiber bundles rather than only lacunes close to midline. Altogether, these results support the hypothesis that the accumulation of lacunes along the fibers of the corpus callosum leads to secondary degeneration translating into corpus callosum atrophy and also slows the connections between brain hemispheres, as illustrated by an increase in reaction time. While we did not observe any significant relationships between the volume of white matter hyperintensities or the number of MB and the corpus callosum area, smaller ischemic lesions, not visible with conventional MR imaging such as microinfarcts, might induce similar changes.
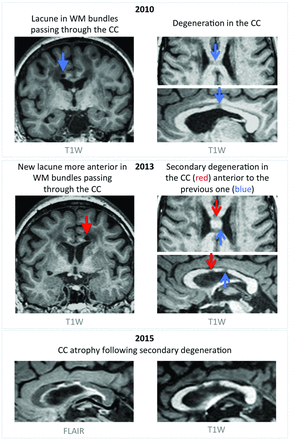
Brain atrophy is often considered a final common pathway adding up the various consequences of vascular lesions accumulating within the brain parenchyma.18 In the present study, patients showed lower CCA but no brain atrophy compared with age- and sex-matched controls. Because of its very simple shape, detection of subtle alterations of the corpus callosum structure might be technically easier than those of the whole brain. However, given the cross-sectional nature of our study, we could not formally exclude the possibility that patients with CADASIL have innate lower CCA compared with healthy subjects, but some arguments contradict this hypothesis. First, in the present study, we found that CCA is independently associated with the volume of lacunes, suggesting that reduction of CCA might be secondary to degeneration of long-range fibers passing through the altered parenchyma, in line with the description of remote cortex atrophy after subcortical infarcts.19 Note that we observed, in 10 of 26 patients, imaging aspects compatible with such secondary changes after the occurrence of lacunes in the corpus callosum fibers (Fig 3). Also, in additional analyses, we compared 28 other patients with CADASIL without lacunes from our national cohort study who were age- and sex-matched to the control group of the present study. When considering the difference in field strengths possibly altering CCA delineation, CCA in patients with CADASIL was not significantly different from that of age- and sex-matched controls (On-line Appendix and On-line Figs 1–5). Altogether, these results strongly support that CCA is actually lower in patients as a consequence of the accumulation of vascular lesions throughout the brain parenchyma and, thus, reflects the disease severity.
Corpus callosum atrophy through the appearance of lacunes and secondary white matter degeneration. An example of a 56-year-old patient with CADASIL presenting in 2010 with hypointense T1-weighted signal in the corpus callosum (CC), presumably as a consequence of a lacune located in white matter bundles passing through the CC body (top panel). In 2013, a new lacune occurred in bundles passing through the CC, localized anterior to the previous one and leading to another hypointense area in the CC (middle panel). Images acquired in 2015 show progressive CC atrophy due to degeneration secondary to the appearance of lacunes (bottom panel). T1W indicates T1-weightd.
Given that CCA was negatively related to the volume of lacunes and positively related to brain atrophy, it might be considered a marker of white matter atrophy. However, several reports have now shown that the presence of lacunes in the white matter is associated with widespread cortical microinfarcts that may themselves explain these relationships independent of direct white matter damage.20 While the results of the present study highlight the potential interest in CCA as a marker of SVD, the mechanisms leading to a smaller CCA in patients with CADASIL remain beyond the reach of our methodology.
The mechanisms leading to reaction time slowing remain largely unknown. While it may be tempting to look deeper into the relationships between reaction time and specific brain structure and/or networks, one must keep in mind that even in healthy subjects, the mechanisms underlying reaction time are strongly debated, with some authors considering it a stochastic process unrelated to specific brain areas or networks.21 Note that the main assumption underlying our results is that reaction time involves efficient interhemispheric cooperation. Further studies including more advanced methods such as assessment of fiber tracts, as previously performed in CADASIL, will be needed to further understand the mechanisms of reaction time slowing in this disorder.22
Processing speed has previously been associated with CCA in sporadic forms of SVD and in other brain disorders.5,23⇓–25 Regarding reports of sporadic forms of SVD, the absence of control subjects did not allow formally disentangling the effects of age from those of SVD in these studies. Moreover, study samples were heterogeneous, including older patients, with a significant proportion probably having associated Alzheimer disease (29% of diagnosed dementia on a 3-year follow-up6). Here, the significantly larger CCA in controls, with no relationship with RT, strongly supports our observation of a specific effect of the disease. Further studies relying, for instance, on diffusion tensor imaging may be of interest to locally quantify the effects of the disease on the corpus callosum structure.
Our study has various limitations. First, study samples were small, including only 26 patients. Given the very strict inclusion criteria of the present study, patient recruitment is challenging and prevents studying large cohorts of patients at this stage of the disease. However, despite small sample sizes, we observed significant and coherent results, emphasizing the strength of the observed relationships. The use of several covariates in multiple regression analyses with such sample sizes may lead to overfitting of the data, but the coherence of our results argues against this hypothesis. More elaborate MR imaging markers, requiring intensive postprocessing methods, might also be related to reaction time. However, we chose to restrict our analyses to the corpus callosum area to provide robust and easy-to-obtain markers for therapeutic trials. Finally, given that most patients had strokes before being prospectively followed, we were not able to determine exactly, for each patient, which of the lacunes initially led to stroke symptoms. We were thus unable to explore separately the relationships between CCA and lacunes that led to stroke and between CCA and “silent” lacunes.
Our study also has strengths. We tested a clear and unique hypothesis with a straightforward methodology, with the use of a simple MR imaging parameter, easily obtained manually and strongly correlated with volumetric measures obtained with methods requiring postprocessing skills. Our patients represent a homogeneous sample of a pure vascular disorder, including patients of various ages, allowing differentiating the effects of disease from those of age. Additionally, the detection of relationships between reaction time and corpus callosum area in small samples implies a large effect size. In patients, the effect of corpus callosum thinning was roughly twice that of aging in controls regarding an increase of reaction time.
Conclusions
We have shown that the CCA is a simple and independent imaging marker of reaction time in the early clinical stages of CADASIL. Given that clinical variability is a well-known characteristic of patients with vascular cognitive impairment, markers such as CCA can appear of high interest for future therapeutic trials in CADASIL, which will likely be performed in patients with the least severe disease. Additional studies are needed to determine whether our results are translatable in the context of sporadic SVD.
Acknowledgments
We thank Chantal Ginisty, Séverine Roger, and Séverine Desmidt (Translational and Applicative Neuroimaging Research Unit, NeuroSpin) for performing the MR imaging acquisitions. We also acknowledge Jocelyne Ruffié, Véronique Joly-Testaud, and Laurence Laurier for the recruitment process of patients and controls. *Money paid to the institution.
Footnotes
This work was funded by a Network of European Funding for Neuroscience Research grant (01EW1207) under the Seventh Framework Programme and the European Research Area Net, with the support of the French-Cerebral Autosomal-Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy Association, the PLANIOL Foundation, the NRJ Foundation, and the Leducq Foundation. The sponsor was Assistance Publique, Hôpitaux de Paris (Département de la Recherche Clinique et du Développement).
Indicates open access to non-subscribers at www.ajnr.org
References
- Received March 1, 2017.
- Accepted after revision June 23, 2017.
- © 2017 by American Journal of Neuroradiology