Abstract
BACKGROUND AND PURPOSE: Although resection of a tumor by trans-sphenoidal surgery is considered the criterion standard for successful surgical treatment of functional pituitary microadenoma, MR imaging occasionally fails to visualize and identify the tumor and supplementary imaging modalities are necessary. We tested the possibility of dynamic contrast-enhanced multisection CT of the pituitary gland accompanying image reconstruction of contrast agent dynamics to identify the localizations of microadenomas and compared the diagnostic performance with conventional pituitary-targeted MR imaging.
MATERIALS AND METHODS: Twenty-eight patients with surgically confirmed functional pituitary microadenomas (including growth hormone–, adrenocorticotropic hormone–, and prolactin-secreting adenomas) who underwent pituitary-targeted dynamic contrast-enhanced multisection CT were retrospectively investigated. We undertook image reconstruction of the dynamics of the contrast agent around the pituitary gland in a voxelwise manner, visualizing any abnormality and enabling qualification of contrast dynamics within the tumor.
RESULTS: Fifteen cases were correctly diagnosed by MR imaging, while dynamic contrast-enhanced multisection CT correctly diagnosed 26 cases. The accuracy of localization was markedly better for adrenocorticotropic hormone–secreting microadenomas, increasing from 32% on MR imaging to 85% by dynamic contrast-enhanced multisection CT. Compared with the normal pituitary gland, adrenocorticotropic hormone–secreting adenoma showed the least difference in contrast enhancement of the different functional microadenomas. Images acquired at 45–60 seconds after contrast agent injection showed the largest difference in contrast enhancement between an adenoma and the normal pituitary gland.
CONCLUSIONS: Dynamic contrast-enhanced multisection CT combined with image reconstruction of the contrast-enhanced dynamics holds promise in detecting MR imaging–occult pituitary microadenomas.
ABBREVIATIONS:
- ACTH
- adrenocorticotropic hormone
- AUC
- area under the curve
- DCE
- dynamic contrast-enhanced
- MCT
- multisection CT
- PRL
- prolactin
- rAUC
- relative AUC
Pituitary microadenoma often shows uncontrolled production of pituitary hormones and causes endocrine disorders such as Cushing disease, acromegaly, and hyperprolactinemia. Although pharmacotherapy has recently played a more pivotal role in treating functional pituitary microadenoma,1,2 resection of the tumor by trans-sphenoidal surgery is still considered the criterion standard.3 Because these tumors tend to be relatively small, precise preoperative identification of a microadenoma is one of the crucial elements for successful surgical treatment of this disease.4
MR imaging with or without contrast agent is most commonly used for this purpose, and dynamic contrast-enhanced techniques are sometimes applied for better tumor visualization.5,6 Moreover, the magnetic field strength typically applied in MR imaging has recently increased from 1.5T to 3T, and clearer imaging of microadenomas has thus been anticipated.4 Such effort, however, often fails to correctly depict the microadenoma, and other modalities such as methionine positron-emission tomography have been suggested to meet this need.4 Methionine PET does indeed hold promise for the visualization of microadenoma but is not yet widely clinically available, and a more clinically accessible technique is necessary for better visualization of this entity. The present study investigated the possibility of dynamic contrast-enhanced multisection CT (DCE-MCT) of the pituitary gland accompanying image reconstruction of contrast agent dynamics to identify the location of a microadenoma and compared the diagnostic performance with conventional pituitary-targeted MR imaging.
Materials and Methods
Patient Characteristics
The selected patients for this study consisted of a consecutive series of all those with endocrinopathy treated by surgery who had undergone both pituitary-targeted dynamic contrast-enhanced multisection CT and MR imaging as presurgical studies. As a result, pituitary-targeted DCE-MCT was performed for 28 patients with functional pituitary microadenoma at Osaka University Hospital between 2004 and 2014 as a preoperative assessment. Patient characteristics are shown in On-line Table 1. The underlying pathology was adrenocorticotropic hormone (ACTH)-secreting adenoma in 13 cases, growth hormone–secreting adenoma in 6, and prolactin (PRL)-secreting adenoma in 9. The institutional review board of the local ethics committee approved research use of the collected data (institutional review board number: 12491), and written consent was waived for this study.
Preoperative MR Imaging
MR imaging was performed at either 1.5T (Signa Genesis/Excite; GE Healthcare, Milwaukee, Wisconsin; or Magnetom Vision Plus; Siemens, Erlangen, Germany) or 3T (Signa HDxt; GE Healthcare; or Achieva/Ingenia; Philips Healthcare, Best, the Netherlands). Six patients were scanned at 1.5T; and 22, at 3T. Standard T1- and T2-weighted images and gadolinium-enhanced T1-weighted images targeting the pituitary gland were obtained. The dynamic contrast-enhanced technique was not included for MR imaging in the current study. Axial, coronal, and sagittal images were routinely obtained for gadolinium-enhanced T1-weighted imaging. Section thickness was 3 mm, with section spacing ranging from 0.3 to 0.6 mm. Detailed parameters for MR imaging are listed in On-line Table 2. The final diagnostic report from board-certified neuroradiologists was referenced for defining the tumor location. The surgeons (M.K., S.O., Y.S.) and the first author (M.K.) confirmed the radiologists' official report by observing the actual MR imaging.
Preoperative Dynamic Contrast-Enhanced Multisection CT
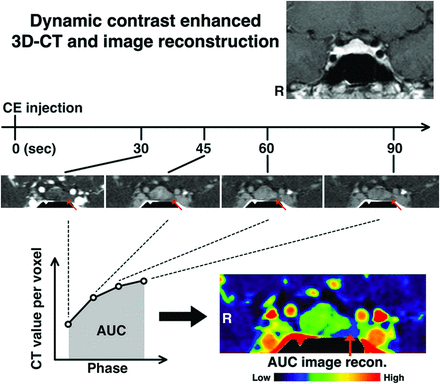
Pituitary-targeted dynamic contrast-enhanced multisection CT was performed by using either a Discovery CT750 HD, LightSpeed Ultra, or LightSpeed VCT system (GE Healthcare). A schematic presentation of the protocol is provided in Fig 1. One hundred milliliters of 300-mg I/mL contrast agent was injected intravenously with an injection rate of 5 mL/s, and MCT was acquired at 30, 45, 60, and 90 seconds after contrast agent injection. MCT was acquired at 60, 90, 120, and 150 seconds after contrast agent injection for 2 cases and at 40, 80, and 120 seconds for 1 case for technical reasons (On-line Table 1). Approximately 3 seconds were required to acquire each phase in a gapless 3D volume. Subsequently, pituitary-targeted axial images were reconstructed at a special resolution of 0.3/0.3/0.6 mm with no section gap.
Schematic presentation for DCE-MCT image acquisition and reconstruction. DCE-MCT was performed at 30, 45, 60, and 90 seconds after contrast agent injection. Subsequently, the “AUC image” was reconstructed in 3D. A representative case of a PRL-secreting pituitary microadenoma (case 20) is illustrated. The red arrows indicate the microadenoma, which was confirmed by surgical removal of the lesion.
Image Reconstruction of Contrast Agent Dynamics and Statistical Analysis
The dynamics of the contrast agent around the pituitary gland were calculated by summation of the acquired multiphase MCT in a voxelwise manner by using software developed in-house on Matlab (MathWorks, Natick, Massachusetts). An ROI was placed preoperatively at the normal pituitary gland and the suspected adenoma by the first author (M.K.) on the reconstructed area under the curve (AUC) images without referring to MR imaging, followed by calculation of ROI statistics. A paired t test, 2-way analysis of variance, or 1-way ANOVA with a Tukey multiple comparison test was performed by using GraphPad Prism software, Version 5.0 (GraphPad Software, San Diego, California).
Trans-Sphenoidal Surgery and Verification of the Adenoma
Judgment of tumor location was preoperatively performed by using both MR imaging and DCE-MCT with AUC-reconstructed images. When MR imaging and DCE-MCT led to conflicting results, a surgical approach to the tumor was planned so that both sides within the sella turcica could be explored. Endoscope-assisted trans-sphenoidal surgery was performed in all cases by 3 neurosurgeons specializing in pituitary surgery (M.K., S.O., Y.S.). Histologic or endocrinologic confirmation was undertaken to confirm the presence or absence of a hormone-secreting functional adenoma at the surgical location.
Results
Diagnostic Efficacy of MR Imaging and DCE-MCT for Functional Pituitary Microadenoma
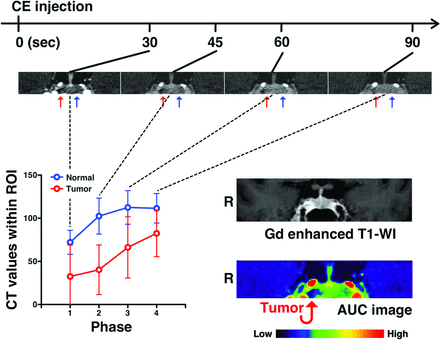
Representative cases are shown in Figs 1 and 2. Figure 1 shows a case of PRL-secreting microadenoma. Contrast-enhanced MR imaging failed to identify tumor within the sella turcica, while DCE-MCT clearly showed decreased and delayed contrast enhancement on the left side of the pituitary gland. Abnormal contrast agent dynamics were much more easily appreciated on the reconstructed AUC image. Figure 2 shows a case of ACTH-secreting microadenoma. Contrast-enhanced MR imaging again failed to identify the presence of tumor, while DCE-MCT along with the reconstructed AUC image clearly suggested a lesion located on the right side of the pituitary gland. Diagnostic performances of MR imaging and DCE-MCT for each type of functional pituitary microadenoma are listed in the Table and On-line Table 1. Overall, 15 of the 28 cases were correctly diagnosed by MR imaging, while DCE-MCT correctly diagnosed 26 cases (Table). The accuracy of location prediction was markedly improved for ACTH-secreting microadenoma, increasing from 32% (4/13) with MR imaging to 85% (11/13) with DCE-MCT.
A representative case of ACTH-secreting pituitary microadenoma. DCE-MCT analysis of an ACTH-secreting pituitary microadenoma (case 12) is presented. Abnormal contrast agent dynamics are observed on the right side of the pituitary gland, though no abnormality is evident on MR imaging. The red arrows indicate the microadenoma, which was confirmed by surgical removal of the lesion. The blue arrows indicate a normal pituitary gland.
Comparison of MRI and CT for correct localization diagnosis of functional microadenomas
Comparison of Contrast-Enhancement Dynamics between the Normal Pituitary Gland and a Functional Pituitary Microadenoma by DCE-MCT
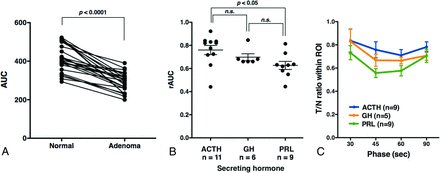
The dynamics of contrast enhancement were compared between the normal pituitary gland and a functional pituitary microadenoma by looking into differences in the AUC retrieved by DCE-MCT. ROIs were placed on either the normal-appearing pituitary gland or the adenoma, the locations of which were confirmed postoperatively. AUC was significantly decreased in the microadenoma compared with the normal pituitary gland (Fig 3A). Relative AUC (rAUC) was subsequently calculated for each lesion, as rAUC = AUCadenoma/AUCpituitary. When contrast-enhanced dynamics are equal between the adenoma and the normal pituitary gland, the rAUC will thus be 1. Fig 3B shows that ACTH-secreting adenomas presented with a significantly higher rAUC compared with PRL-secreting adenomas, and the rAUC of ACTH-secreting adenoma was close to 1. A trend was also seen for the growth hormone–secreting adenoma to show lower rAUC than the ACTH-secreting adenoma. These results suggest that the contrast-enhanced dynamics of ACTH-secreting microadenomas are relatively similar to those of the normal pituitary gland compared with PRL- or growth hormone–secreting microadenomas. This finding was also confirmed by analyzing the ratio of contrast enhancement compared with the normal pituitary gland in each phase during DCE-MCT. The ACTH-secreting adenoma showed the least contrast-enhancement differences compared with the normal pituitary gland (Fig 3C). These differences were significant (P = .01, 2-way ANOVA). In addition, the time phase that showed the largest difference in contrast enhancement between the adenoma and the normal pituitary gland was 45–60 seconds after contrast agent injection, irrespective of the secreted hormone.
Contrast agent dynamics of pituitary microadenomas assessed by AUC. A, Adenomas show significantly lower AUC compared with the normal pituitary gland (P < .0001, paired t test). B, ACTH-secreting pituitary microadenomas show significantly higher rAUC compared with PRL-secreting microadenomas (P < .05, 1-way ANOVA with a Tukey multiple comparison), suggesting that contrast agent dynamics within ACTH-secreting microadenomas are similar to those of the normal pituitary gland. GH indicates growth hormone. C, The ratio of CT values of adenomas to those of the normal pituitary gland (tumor/node ratio [T/n ratio]) is plotted as a function of the time phase during DCE-MCT. Twenty-three cases in which CT acquisition was performed at 30, 45, 60, and 90 seconds were collected. The most significant drop was observed at 45–60 seconds, irrespective of the secreted hormone. In addition, ACTH-secreting adenomas showed the highest tumor/node ratio among the 3 hormones, indicating the least contrast between the adenoma and normal pituitary gland (2-way ANOVA, P = .01).
Discussion
Successful surgical treatment of functional pituitary microadenoma largely relies on accurate identification of the tumor within the sella turcica.4 These relatively small tumors represent a challenge to both neuroradiologists and neurosurgeons in locating them, resulting in a greater potential for insufficient treatment of the lesion. The criterion standard technique used for lesion localization is MR imaging,5⇓–7 and some clinical investigations have suggested contrast-enhanced CT,8,9 super-selective venous sampling of pituitary hormone levels,10⇓–12 and methionine PET4 as useful modalities to supplement MR imaging findings. The clinical values of these additional presurgical studies, however, remain undetermined, and conflicting results have been reported. For example, one report has claimed that venous sampling of ACTH at the inferior petrosal sinus is informative for determining adenoma location,12 while others have reported results to the contrary.11 Methionine PET has also been proposed as a promising imaging technique to identify MR imaging–occult ACTH-secreting microadenomas. MR imaging–registered methionine PET was previously reported as showing superb performance in detecting ACTH-secreting microadenomas, of which identification was significantly difficult by using MR imaging alone.4 The availability of methionine PET, however, remains limited, and more extensive studies are required to confirm the clinical value of methionine PET for diagnosing functional microadenoma.
MR imaging shows several technical limitations in elucidating the presence of microadenoma. The above-mentioned small size of the tumor is one. To guarantee sufficient image quality, we usually select a section thickness of 3 mm for pituitary imaging. Given the sizes of microadenomas, which are <10 mm, there is a high chance of overlooking the lesion. In addition to the problem of size, pituitary adenoma imaging by using a contrast agent largely relies on the adenomas showing much less contrast enhancement than the normal pituitary gland. As Fig 3B suggests, an ACTH-secreting microadenoma, in particular, shows contrast-enhanced dynamics similar to that of the normal pituitary gland, which seems likely to contribute to failed detection of the lesion on MR imaging. Although the dynamic contrast-enhanced technique is often applied on MR imaging to overcome this issue, scanning time usually required to obtain each dynamic phase ranges from 20 to 30 seconds6 or is shortened into 12–20 seconds in some cases, but it is not possible to obtain a gapless 3D image as in MCT. Figure 3C, in particular, highlights this problem. The most suitable time phase to obtain sufficient contrast between an adenoma and the normal pituitary gland is 45–60 seconds after contrast agent injection. This adenoma/normal pituitary gland contrast will rapidly diminish within the subsequent 30 seconds. Both spatial and temporal resolution must, therefore, be sufficiently high to visualize the presence of the adenoma.
The proposed CT-based imaging technique has the potential to overcome these technical difficulties associated with MR imaging, mainly due to the superior temporal resolution of CT compared with MR imaging. Each phase of MCT can be acquired in a full 3D image within 3 seconds, which provides satisfactory spatial and temporal resolution. The idea of using DCE-MCT for microadenoma detection has been proposed before.8,9 To better visualize contrast-enhancement dynamics in a voxelwise manner, the present study applied image reconstruction. The resulting AUC images provided intuitive images for clinicians to identify lesions with abnormal contrast-enhancement dynamics.
Limitations of the current study should be mentioned. First, this study was not a direct comparison between DCE–MR imaging and DCE-MCT. The patient cohort for this study did not have DCE–MR imaging as presurgical imaging for pituitary microadenomas. Further study is necessary to critically evaluate the clinical value of DCE-MCT with an image-reconstruction technique compared with conventional DCE–MR imaging. Another concern is the MR image quality of the current study. Previous studies reported exhibiting 66%–100% sensitivity in detecting ACTH-secreting microadenoma6,7 with the aid of DCE–MR imaging. The sensitivity of the current study for detecting ACTH-secreting microadenomas was as low as 32%, which may suggest that MR images of the current study might have been suboptimized compared with the past literature reports. In addition, although it is intriguing to contemplate why ACTH-secreting microadenomas show different contrast-enhanced dynamics compared with other functional microadenomas as shown in Fig 3C, the pathology of the blood supply to microadenomas is unfortunately not yet well-understood, making it difficult to reach any conclusive argument on this matter.
In summary, the present results show that DCE-MCT images along with AUC images can help identify microadenomas and improve the overall detection of those lesions compared with MR imaging alone. Although this study was not a direct comparison between DCE–MR imaging and DCE-MCT, it seems valid to conclude that DCE-MCT is a noninvasive diagnostic technique, which, along with the reported AUC reconstruction method, could be recommended as a supplementary diagnostic technique for MR imaging–occult functional microadenoma.
Conclusions
Dynamic contrast-enhanced multisection CT combined with image reconstruction of the contrast-enhanced dynamics holds promise in detecting MR imaging–occult pituitary microadenoma. Because surgical outcomes are highly reliant on accurate preoperative identification of the adenoma, the proposed technique should contribute to better surgical outcomes for functional pituitary microadenomas.
Footnotes
Disclosures: Manabu Kinoshita—RELATED: Grant: Research Grant from the Life Science Foundation of Japan,* Scientific Research (C) from the Japan Society for the Promotion of Science,* Research Grant from the SENSHIN Medical Research Foundation,* Research Grant from the Aichi Cancer Research Foundation*; UNRELATED: Grants/Grants Pending: Research Grant from the Japanese Foundation for Multidisciplinary Treatment of Cancer.* Yoshiyuki Watanabe—UNRELATED: Grants/Grants Pending: Toshiba Medical Japan,* Comments: research fund about 320-row CT; Payment for Lectures (including service on Speakers Bureaus): GE Healthcare, Bayer Pharmaceutical. Youichi Saitoh—UNRELATED: Consultancy: Teijin Pharma; Payment for Lectures (including service on Speakers Bureaus): Teijin Pharma. *Money paid to the institution.
This work was supported by the Aichi Cancer Research Foundation, the SENSHIN Medical Research Foundation, the Life Science Foundation of Japan, and the Japan Society for the Promotion of Science KAKENHI (25462256).
Indicates open access to non-subscribers at www.ajnr.org
REFERENCES
- Received September 24, 2014.
- Accepted after revision November 2, 2014.
- © 2015 by American Journal of Neuroradiology