Abstract
SUMMARY: In this study, we compared lesion size by using VADC and VT2 at 0, 2, 5, 24, and 48 hours and histologic lesions at 48 hours in a P7 rat stroke model. The best correlation between VHISTO and VADC was at H0, and between VHISTO and VT2, at H2-H5. Early MR imaging signals allowed excluding “no-lesion” and “no-reflow” animals to help standardize this neonatal stroke model and predict lesion size.
ABBREVIATIONS:
- H0, H2, H5, H24, and H48
- 15 minutes and 2, 5, 24, and 48 hours after reperfusion, respectively
- P7
- 7-day-old
- RARE
- rapid acquisition relaxation excitement
- VADC
- volume of lesion on ADC maps
- VHISTO
- volume of lesion on histologic sections
- VT2
- volume of lesion on T2 maps
Neuroimaging, particularly MR imaging, could be a tool for a rapid comprehensive assessment in acute stroke with the potential to guide treatment decisions for a better clinical outcome. One of the key issues in stroke assessment is to choose the best end points that appropriately indicate outcome. In experimental animal research, final lesion size has been used consistently as an outcome. Infarct sizes assessed by T2WI have been shown to correlate well by some and to underestimate histologic damage by others.1 In the developing brain, authors have reported that MR imaging in P7 rat pups submitted to stroke2 or hypoxia-ischemia3,4 seems correlated to final histologic lesions measured 24 hours or 3–10 days after the injury, without early MR imaging time-point evaluation.
The aim of the present study was to develop a preclinical design including routine MR imaging coupling ADC maps, T2 maps, and MRA. We suggested that this strategy could improve the statistical power of trials using our model by diminishing the variability of the outcome assessment. Early MR imaging could 1) confirm initial ischemia, 2) confirm arterial reflow, and 3) accurately predict the final histologic outcome at 48 hours.
Technique
Perinatal Ischemia
Care and use of experimental animals followed French and European Community guidelines. Ischemia was created in 24 Wistar P7 rats (Janvier, Le Genest Saint Isle, France) of both sexes.5 Briefly, anesthetized (chloral hydrate, 350 mg/kg, intraperitoneally) rats were exposed to left MCA electrocoagulation followed by 50-minute transient left common carotid artery occlusion. During ischemia and recovery the pups were placed in a humidified incubator at 37°C.
MR Imaging Acquisition
Pups were monitored at H0, H2, H5, H24, and H48 after reperfusion. For H0, H2, and H5 imaging session, pups were asleep due to the initial chloral hydrate injection. For H24 and H48 imaging sessions, pups were anesthetized by isoflurane 0.8% mixed in 30% O2, 70% N2. During imaging, animals were thermoregulated by a water blanket heated at 37°C. Images were acquired by using a 7T horizontal magnet equipped with a Bruker AVANCE console and with an actively shielded gradient 360mT device (Bruker BioSpin, Rheinstetten, Germany). DWI was performed from an EPI sequence (TR = 3 seconds, TE = 25 ms, b-values = 50, 500, 1000 s/mm2, FOV = 40 × 40 mm, matrix = 256 × 256, repetitions = 3, 25 sections, thickness = 0.5 mm, duration = 9.5 minutes). T2WI was performed with a RARE sequence (TR = 5404 ms, TE values = 25.6, 76.8, 128, 179.2 ms, RARE factor = 4, FOV = 40 × 40 mm, matrix = 128 × 128, 25 sections, thickness = 0.5 mm, duration = 3 minutes). ADC and T2 maps were computed by exponential fitting of the local signal-intensity versus b and TE, respectively, by using a homemade ImageJ plugin (http://rsbweb.nih.gov/ij/). MRA was performed with a 3D time-of-flight method based on a fast low-angle shot 3D sequence (TR = 15 ms, TE = 2.5 ms, FOV= 40 × 40 × 40 mm, matrix = 256 × 256 × 128, flip angle = 20°, duration = 3 minutes). Each animal underwent the H0 imaging and was then eventually excluded either as “no-lesion” if the DWI lesion was visible on <3 sections or as “no-reflow” if MRA showed an ipsilateral ICA signal of <40% of the contralateral ICA signal. All animals were sacrificed immediately after the H48 imaging session.
Outcome Assessments
The volume of the lesion was assessed by using histology at H48, ADC maps, and T2 maps from H0 to H48. VHISTO was delineated by a classic visual method on cresyl violet–stained coronal sections (50-μm thick, 500-μm intervals). VADC and VT2 were defined by applying a threshold (mean − 2 SDs) of the whole contralateral hemisphere ADC and T2, respectively. Volume measurements were expressed as a percentage of the ipsilateral hemisphere.
Statistical Analysis
All results are given as mean ± SD. The correlation and regression were performed with a standard statistical software package (SSYSTAT 3.1, Systat Software, San Jose, California) by using a Spearman rank-order correlation and a linear regression, respectively.
Results
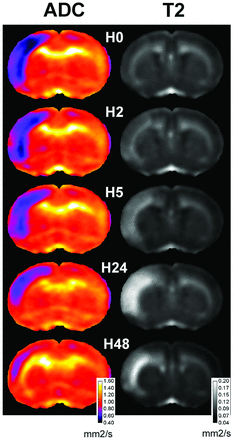
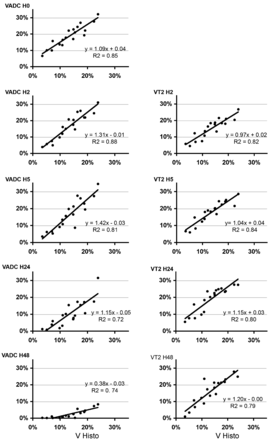
Four animals of 24 (17%) were excluded as no-lesion. All exhibited MCA interruption on H0 angiography, without a significant DWI lesion. Two animals of 24 (8%) were excluded as “no-reflow” as determined on MRA. Eighteen animals were then analyzed (Fig 1), and VADC, VT2, and VHISTO were calculated (Fig 2). An area in the ipsilateral frontoparietal cortex with low VADC was maximal at H0 in almost all the animals (Fig 1). VADC at H0 was different from 6.6%–32.1% of the ipsilateral hemisphere. A significant correlation between VADC at H0 and lesion volume (VHISTO) at 48 hours was obtained (slope: 1.09, r = 0.92, P < .001; Fig 2). In some animals, VADC slightly decreased between H2 and H5, whereas it remained similar in the others. At 24 and more markedly at 48 hours, all VADC values were highly reduced (0.2%–8.3%; Fig 1). Even if hints in the deeper cortical and subcortical layers (Fig 1) were visible on T2 maps as soon as H0 (in few animals), the T2 lesion was clearly identifiable in all animals at H2 (4.4%–26.7%). A significant correlation between VT2 and VHISTO was obtained at H2 (slope: 0.97, r = 0.91, P < .001) and H5 (slope: 1.04, r = 0.91, P < .001; Fig 2). This correlation remained at H24 but with a higher slope (1.15, r = 0.89). Then VT2 slightly decreased at H48 (r = 0.888). The range of VHISTO was from 3.5% to 24% with a mean of 13.5 ± 5.7%.
Time course of ADC and T2 maps at Bregma 1.20 mm. Sum of coregistrated sections are computed at H0, H2, H5, H24, and H48. Note that low ADC was observed in the lesioned frontoparietal cortex compared with the contralateral side. Hints of T2 were observed as early as H0 in the deeper cortical layers. At H48, marked T2 signals are still detected in the deeper cortex.
Correlation between MR imaging and histology measurements. VHISTO and VADC (left) or VT2 (right) are given at different time points after reperfusion. Note that correlation was very strong at H0 on the ADC map and at H2 and H5 on the T2 maps.
Discussion
This study showed that routine MR imaging could be a toolbox able to standardize neonatal stroke models, first by excluding no-lesion and no-reflow animals in the first MR angiography, and second by predicting histologic outcome early in a neonatal stroke model.
The first set of MR images at H0 was used to exclude no-lesion animals. These animals probably developed efficient cortical arterial anastomoses during ischemia as we previously demonstrated by using sonography.6 MR imaging also allowed excluding no-reflow animals because the absence of recanalization was shown to block the development of further edema.7
All animals exhibited a quite reproducible time course of ADC or T2 volume. As previously reported, VADC reached a maximum just after reperfusion.7 The best-correlated VADC time point with VHISTO was H0. This impressive correlation lets one think that as soon as 15 minutes after reperfusion, “the die was cast,” and almost the whole interindividual variability of animals was already determined. In contrast, at H2 and H5, the slope of regression was superior to 1, suggesting that VADC overestimated histology volume. Conversely, at 24 and 48 hours, correlation between ADC and histology became less strong and the slope coefficient decreased, leading to an underestimation of the final lesion.
As previously reported, T2 lesions appeared as soon as 2 hours after ischemia.7 Most interesting, we observed that the correlation of VT2 with VHISTO was strong as early as H2 and H5, with a slope of regression close to 1, suggesting good overlap between T2 signals and histologic lesions. Thereafter, VT2 overestimated lesions at both 24 and 48 hours.
Conclusions
This whole strategy was not reported in the literature because only parts of studies reported exclusion of no-lesion animals with intraischemic MR imaging.8 In addition, early MR imaging signals appear to be good surrogates for final stroke lesions in the developing brain.
Footnotes
Disclosures: Christiane Charriaut-Marlangue—RELATED: Support for Travel to Meetings for the Study or Other Purposes: Société Française sur la Recherche du Handicap de l'Enfant.* Sébastien Fau—RELATED: Grant: CNRS, Comments: French National Scientific Research Center. *Money paid to the institution.
This study was supported by the Institut pour la Recherche sur la Moelle Epinière, Fondation NRJ and Société Française sur la Recherche du Handicap de l'Enfant. S. Fau was the recipient of a “poste d'accueil“ from AP-HP and CNRS.
C.C.M., S.F., and P.M. designed and organized this study; S.F., C.P., and C.G. performed the experiments and analyzed data. C.C.M., S.F., and C.P. wrote the manuscript.
Paper previously presented as a poster at: Brain 09, 24th International Symposium on Cerebral Blood Flow, Metabolism, and Function, Chicago, Illinois, June 29–July 3, 2009.
Indicates open access to non-subscribers at www.ajnr.org
REFERENCES
- Received December 6, 2011.
- Accepted after revision January 6, 2012.
- © 2013 by American Journal of Neuroradiology