Abstract
BACKGROUND AND PURPOSE: DTI is widely used for the evaluation of white matter integrity in various neurologic diseases. The purpose of this study was to investigate changes in white matter integrity by using DTI in NAWM of patients with MMD and to evaluate the correlation between diffusion and perfusion characteristics through an interhemispheric comparison.
MATERIALS AND METHODS: We retrospectively reviewed 20 primary MMD patients with asymmetric disease stage and 20 age-matched healthy controls. FACS and ADCCS values of bilateral centrum semiovale were measured by using region of interest analysis. Mean FACS and ADCCS were compared between patient and control groups by unpaired t test. Interhemispheric differences in FACS and ADCCS were assessed and compared between the H-TTPdelayed and the H-TTPshorter by using paired t test. AIs also were assessed to verify interhemispheric differences.
RESULTS: The patient group showed a significantly lower mean FACS and a higher mean ADCCS value than the control group. In the patient group, the H-TTPdelayed had a significantly lower FACS and higher ADCCS value than the H-TTPshorter. Both AIFA and AIADC were significantly higher in the patient compared with the control group.
CONCLUSIONS: DTI can describe subtle changes in white matter integrity in NAWM of patients with primary MMD that are not detected by conventional MR imaging. In addition, diffusion characteristics are well correlated with perfusion characteristics. We believe that DTI is a useful ancillary tool to evaluate patients with MMD.
Abbreviations
- ADCCS
- apparent diffusion coefficient in centrum semiovale
- AI
- asymmetric index
- FACS
- fractional anisotropy in centrum semiovale
- H-TTPdelayed
- hemisphere with more delayed time-to-peak
- H-TTPshorter
- contralateral hemisphere of H-TTPdelayed
- MMD
- Moyamoya disease
- NAWM
- normal-appearing white matter
- PWI
- perfusion-weighted MR imaging
- rCBV
- relative cerebral blood volume
MMD is a chronic steno-occlusive disease of the internal carotid arteries that mainly affects children. Various imaging techniques have been applied to evaluate the hemodynamic status, tissue integrity, and prognosis of MMD.1–3
The recent development of DTI has allowed visualization of fiber orientation and microstructural integrity in white matter and is considered to be a very useful tool for evaluating white matter in various central nervous system diseases.4–7 DTI has been accepted as a tool with sufficient sensitivity to detect brain pathologies hidden in NAWM.8–11 For example, DTI can reveal microstructural damage of NAWM in patients with chronic cerebral arterial occlusive disease.11,12
In patients with MMD, progressive vascular stenosis causes chronic cerebral hypoperfusion in the territory of the internal carotid arteries, but partial adaptation occurs through collateral blood flow from the posterior circulation, the ethmoidal vessel, and other basal vessels.13 MMD with partially adapted chronic hypoperfusion follows a dynamic disease process. Patients with MMD may experience serial ischemic attacks, and some patients eventually experience cerebral infarct. We hypothesized that NAWM in MMD patients without cerebral infarct may be affected by chronic hypoperfusion, and DTI could reveal cumulative microstructural damage that is not visualized by conventional MR imaging.
Perfusion MR imaging has been found to be effective in the evaluation of cerebral hemodynamics in MMD.3,14–16 TTP images are simple to use for the assessment of collateral status, development of neovascularization after encephaloduroarteriosyangiosis, and general perfusion status. In addition, the ischemic hemisphere usually shows an increased rCBV due to compensatory vasodilation and delayed TTP due to proximal vessel stenosis.17 In MMD without infarction, an interhemispheric difference in TTP may be present due to different stages of proximal vessel stenosis. We hypothesized that the hemisphere with more delayed TTP (H-TTPdelayed) may be vulnerable to hypoperfusion, so NAWM in the H-TTPdelayed may demonstrate a higher level of cumulative microstructural damage than NAWM in the contralateral hemisphere.
Thus, the purpose of this study was to investigate changes in white matter integrity by using DTI in NAWM of patients with MMD and to evaluate the correlation between diffusion and perfusion characteristics by an interhemispheric comparison.
Materials and Methods
Patients and Control Subjects
The institutional review board approved this retrospective study and waived the requirement for informed consent from patients or legal guardians. We retrospectively reviewed brain MR images and clinical data of 103 childhood MMD patients from August 2006 to August 2009 at the Severance Hospital of the Yonsei University Health System of Korea. Of these patients, 20 (15 girls and 5 boys; mean age, 9.55 years; range, 5–15 years) who satisfied the following criteria were included in the patient group: 1) patients who were diagnosed with primary childhood MMD on MR imaging and MRA according to the diagnostic criteria and guidelines for MMD proposed by the Research Committee on Spontaneous Occlusion of the Circle of Willis18; 2) patients who simultaneously underwent FLAIR imaging, MRA, PWI imaging, and DTI; 3) patients who had no clinical history of therapeutic intervention, such as pharmacotherapy (vasodilator or anticoagulant) or surgery (bypass or indirect revascularization procedure) before undergoing MR imaging; 4) patients who had no overt cerebral infarct on conventional MR imaging; and 5) patients who showed definitive asymmetric involvement upon visual analysis of MRA and TTP maps. Thirty-seven patients satisfied criteria 1–4, and 20 among them satisfied criteria 5 and were therefore included in the final patient group.
Because healthy childhood volunteers were not available due to the sedation procedure, “closest-to-normal” control diffusion data were obtained from 20 age-matched children (7 girls and 13 boys; mean age, 9.75 years; age range, 5–15 years) who had no vascular pathology on either radiologic or clinical evaluation. The control subjects had the following indications for MR imaging: central nervous system symptoms (headache, 12 patients; seizure, 2 patients) or psychiatric problems (schizophrenia, 2 patients; social phobia, 2 patients; adjustment disorder, 1 patient; conversion disorder, 1 patient). Conventional MR imaging and DTI were simultaneously performed on controls during the same period as for patient subjects.
Data Acquisition
All examinations were performed by 3T MR imaging (Achieva; Philips Medical Systems, Best, the Netherlands) by using an 8-channel sensitivity-encoding head coil. DTI, PWI, MRA, FLAIR, T2-weighted, and gradient-echo T2*-weighted images were obtained in patients with MMD. T1-weighted, T2-weighted, and postcontrast T1-weighted images and DTI were obtained in the control group.
DTI was performed by using single-shot spin-echo-EPI. The axial images were obtained parallel to the anterior/posterior commissure line. The parameters for DTI were as follows: TR/TE/flip angle = 6000 ms/100 ms/90°, FOV = 22 cm, acquisition matrix = 128 × 128, section thickness/intersection gap = 5 mm/2 mm, and NEX = 4. Diffusion sensitizing gradient encoding was applied in 6 directions (x, y, z, xy, yz, and zx) with b at 1000 seconds/mm2, and 1 image was acquired without by using a diffusion gradient (with b at 0). Isotropic ADC, trace, and FA maps were immediately generated on the console by research software (Packman Tools; Philips Medical Systems). The 6 elements of the diffusion tensor were estimated in each voxel assuming a mono-exponential relationship between signal intensity and the b-matrix. Using multivariate regression, the eigenvectors and eigenvalues of the diffusion tensor were determined. To minimize the eddy current–related artifact, we implemented zero and first-order eddy current compensations into the MR imaging system and monitored and calibrated the eddy current level.
PWI was carried out by using multishot gradient-echo principles of echo shifting with a train of observations technique by using the following parameters: TR/TE/flip angle = 2000/60/90°, matrix = 128 × 128, FOV = 24 cm, and section thickness/intersection gap = 5 mm/2 mm. All 60 phase images were obtained before, during, and after the administration of contrast agent (0.1 mmol of gadopentetate dimeglumine [Magnevist; Schering, Berlin, Germany] per kilogram of body weight at a rate of 2 mL/s) through an MR imaging–compatible power injector (Spectris; Medrad, Indianola, Pennsylvania) in those patients older than 5 years or by manual injection in those patients 5 years and younger. This injection was followed by a 15-mL bolus of saline administered at the same injection rate. Perfusion maps of TTP and CBV were generated after eliminating the effect of contrast agent recirculation by γ-variate curve fitting.19,20
MRA was obtained with the 3D time-of-flight technique with a 3D spoiled gradient recalled-echo sequence by using the following parameters: TR/TE/flip angle = 24/3.45/20°, matrix = 512 × 208, FOV = 20 cm, section thickness = 0.8 mm, and effective voxel size = 0.39 × 0.96 × 0.8 mm.
Conventional T1-weighted (TR/TE/flip angle, 2000 ms/10 ms/90°), FLAIR (TR/TE/TI, 11,000 ms/125 ms/2800 ms), T2-weighted (TR/TE, 3000 ms/80 ms), and gradient-echo T2*-weighted (TR/TE/flip angle, 576 ms/15 ms/18°) images were obtained by using the respective parameters.
Data Analysis
In each MMD patient, the H-TTPdelayed and the greater degree of proximal vessel stenosis was determined by the consensus of 2 radiologists (H.J. with 3 years of experience in MR imaging interpretation and S.-K.L. with 13 years of experience) by visual analysis of the TTP map and by using the MRA scoring system proposed by Houkin et al.21 The rCBV patterns in the areas with more delayed TTP also were visually evaluated.
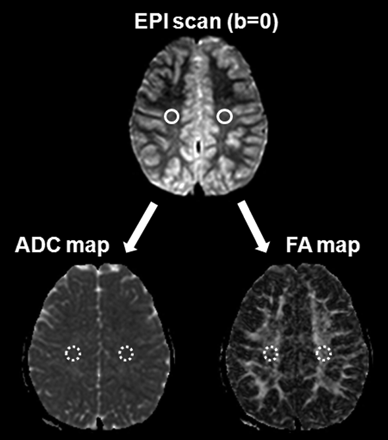
ADC and FA values were measured in the bilateral centrum semiovale of both patient and control groups. The software program NeuRoi (Dr C. Tench, Department of Clinical Neurology, University of Nottingham, United Kingdom), was used for semiautomatic measurement. This program is available at www.nottingham.ac.uk/scs/divisions/clinicalneurology/index.aspx. The ROIs in the centrum semiovale were drawn manually in a 60-mm3 round shape. To avoid contamination from adjacent CSF and gray matter, the ROIs were drawn on the EPI scan of the b = 0 step (T2-weighted, but not diffusion-weighted) at the level of the centrum semiovale, and then automatically overlaid onto the coregistered FA and ADC images (Fig 1). To ensure consistency and consensus, 1 investigator (H.J.) drew the ROIs and 1 board-certified neuroradiologist (S.-K.L.) subsequently confirmed them.
ROI method for ADCCS and FACS measurement. Round ROIs (60 mm3) were drawn on the EPI scan of the b = 0 step at the level of the centrum semiovale, followed by automatic overlaying onto the coregistered FA and ADC images.
Statistical Analysis
We compared the FA and ADC values between the patient and control groups by using the independent samples t test. We then compared the diffusion characteristics of H-TTPdelayed and the H-TTPshorter in the patient group by using the paired samples t test.
Because diffusion characteristics in the normal brain are somewhat asymmetrical,22 AIs also were used to verify the significance of the difference between H-TTPdelayed and H-TTPshorter. The mathematic equations providing AI for FA values in the centrum semiovale were as follows:

 where FA-H-TTPshorter is the FA value in the H-TTPshorter (FA-H-TTPshorter), FA-H-TTPdelayed is the FA value in the H-TTPdelayed (FA-H-TTPdelayed), FARt is the FA value in the right hemisphere, and FALt is the FA value in the left hemisphere.
where FA-H-TTPshorter is the FA value in the H-TTPshorter (FA-H-TTPshorter), FA-H-TTPdelayed is the FA value in the H-TTPdelayed (FA-H-TTPdelayed), FARt is the FA value in the right hemisphere, and FALt is the FA value in the left hemisphere.
The same pattern of mathematic equations also was applied to the ADC values. The independent samples t test was used to compare AI between patient and control groups.
A probability of <5% was considered statistically significant. All statistical analyses were performed by using MedCalc 7.4.0.0 software (MedCalc, Mariakerke, Belgium).
Results
The clinical information and findings pertaining to the hemisphere with TTPdelayed and greater degree of proximal vessel stenosis among the bilateral hemispheres, and the rCBV pattern are summarized in Table 1. Fourteen of the 20 MMD patients presented with recurrent TIA. In patients with recurrent TIA, H-TTPdelayed was highly concordant with the symptomatic hemisphere (95%). The detailed FA and ADC values in each group are summarized in Table 2.
Clinical information and findings for TTP status, degree of supraclinoid ICA or MCA stenosis, and rCBV pattern
Measured diffusion characteristics in patient and control groups
The patient group showed a significantly lower mean FACS value and a higher mean ADCCS value compared with the control group (Table 3). The results of the interhemispheric comparison are summarized in Table 4. In the patient group, H-TTPdelayed showed a significantly lower FACS value and a higher ADCCS value compared with H-TTPshorter (Fig 2). There were no significant differences in the mean FA and ADC values between the right and left centrum semiovales in the control group. Both AIFA and AIADC were significantly higher in the patient group compared with the control group.
Comparison of diffusion characteristics between patient and control groups
Interhemispheric comparison of diffusion characteristics
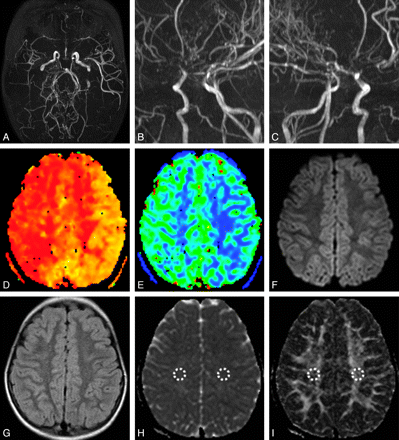
Primary MMD in a 6-year-old girl. A–C, MRA images showed asymmetric MRA cores and stages between both hemispheres (Rt., stage III; Lt., stage II). TTP (D) and CBV (E) maps demonstrated asymmetric hemodynamic status, in which the Rt. hemisphere showed more delayed TTP and increased CBV. Diffusion-weighted image (b = 1000; F) and a FLAIR image (G) did not show any abnormalities. H, ADC map, right side ADCCS (836.3 mm2/s) was higher than left side ADCCS (779.8 mm2/s). I, FA map, right side FACS (0.438 × 10−3) was lower than left side FACS (0.487 × 10−3).
Discussion
We demonstrated that DTI could detect the cumulative microstructural damage that was not detected on conventional MR imaging in the NAWM with chronic hypoperfusion in primary childhood MMD without overt infarction. In addition, we revealed that the NAWM of the hemisphere with more delayed perfusion in each MMD patient had more microstructural damage.
The 2 most commonly determined diffusion properties are the FA, reflecting the degree to which the diffusion tensor deviates from isotropy, and the ADC, reflecting the orientation-averaged apparent diffusivity.7 The FA value may be low when white matter integrity is disrupted. Several studies revealed that the FA value is an indicator of tissue damage in the white matter despite the absence of definite abnormalities on conventional T2 or FLAIR images.11,12,23 The net diffusion of water molecules measured in a particular tissue is referred to as ADC.5 In chronic hypoperfusion, ischemic injury to white matter is associated with axonal destruction and glial proliferation.24 These microstructural changes are associated with an increase in the ADC, probably reflecting increased water diffusivity due to axonal loss.25 However, ADC changes are not confined to ischemic lesions and may occur in NAWM.11,26–28 Thus, we investigated changes in integrity of NAWM with MMD, by using measurements of both FA and ADC.
Our results showed that NAWM of MMD patients without infarction had a significantly lower mean FACS value and a higher mean ADCCS value compared with controls. There have been a few previous DTI studies for MMD. Using whole brain histogram analysis, Mori et al29 revealed that MMD with infarction had significantly lower FA and mean diffusivity than controls. Moreover, they reported that MMD without infarction showed the same tendency, though the differences were not statistically significant. The discrepancy in statistical significance between our study and that of Mori et al29 can be explained by differences in data analysis. The whole brain histogram is an accurate and robust method for overall measurements, but it cannot reveal results in a specific site. We therefore assume that in our study the localized regional analysis for centrum semiovale revealed significant differences in diffusion characteristics, suggesting microstructural damage in the NAWM of patients with MMD without infarction. Recently, Conklin et al30 revealed that regions of steal phenomenon are spatially correlated with elevated ADC in NAWM of patients with MMD, suggesting that even if there is no overt infarction in MMD, microstructural damage of NAWM can develop from low-grade ischemia. Our finding of increased ADC in NAWM of patients with MMD without infarction is consistent with the findings of Conklin et al.30 We found a significantly lower mean FACS value and a higher mean ADCCS value in patients with MMD, probably reflecting cumulative white matter damage from chronic hypoperfusion and recurrent ischemic attacks.
Next, we analyzed the interhemispheric DTI difference in patients with MMD who showed asymmetric TTP status. MMD was defined as bilateral disease, but MMD is known to have a rather asymmetric perfusion appearance, especially in children. MMD also can show a somewhat asymmetric disease status in chronic progressive disease. This asymmetric disease status in MMD can be detected on MRA and PWI. In MMD, the hypoperfused hemisphere usually shows increased rCBV due to compensatory vasodilation, and delayed TTP represents proximal occlusion or stenosis and collateral flow. Our results showed that the hemisphere with a more delayed TTP and a higher MRA score had a significantly lower FACS value and a higher ADCCS value than the more perfused contralateral hemisphere. This result may reflect that NAWM of H-TTPdelayed is more vulnerable to hypoperfusion than the more perfused contralateral hemisphere and that NAWM in H-TTPdelayed may demonstrate more cumulative microstructural damage than NAWM in the contralateral hemisphere.
We note that our study has some limitations. First, the retrospective data analysis resulted in a reduced number of enrolled patients with a high degree of homogeneity in terms of disease and age. Second, for ethical reasons, ideal healthy control subjects were not recruited and structurally normal children's brains were used for comparison. However, we speculate that our results would not have been too different if this study had been performed with ideal controls. Third, DTI in this study was performed with only 6 different diffusion gradients. It has been reported that at least 20 diffusion gradients are necessary for a robust estimation of diffusion characteristics.31 The small number of diffusion gradients in our study could thus be a limitation. Fourth, the lack of absolute quantification in perfusion MR imaging is another limitation. Thus, future investigation by using quantitative analysis of PWI and DTI with at least 20 diffusion gradients is needed.
Conclusions
DTI can detect subtle changes in white matter integrity in chronic hypoperfused brains of patients with primary childhood MMD that are not detected by conventional MR imaging. In addition, diffusion and perfusion characteristics are well correlated. Our findings suggest that DTI can be used as an ancillary tool to evaluate disease severity of patients with primary childhood MMD.
References
- Received February 28, 2011.
- Accepted after revision March 10, 2011.
- © 2011 by American Journal of Neuroradiology