Abstract
BACKGROUND AND PURPOSE: Thromboembolism is a recognized complication occurring during endovascular coil embolization of intracranial aneurysms. Recently, there has been much interest in glycoprotein IIb/IIIa inhibitors to treat such complications, but the evidence is limited. We reviewed our use of one such agent, abciximab, which we commonly administer and believe to be a safe and suitable rescue agent in this setting.
MATERIALS AND METHODS: We retrospectively reviewed cases in which abciximab was administered in our institution between 2001 and 2007. Clinical outcome was assessed by the modified Rankin Scale (mRS) at 6 months. Good outcome was defined as no significant clinical sequelae compared with baseline status or clinical improvement (mRS < 2). Poor outcome was defined as no resolution of a new clinical deficit that developed postprocedure at 6 months (mRS > 2). Angiographic appearance of thromboembolic phenomena and posttreatment outcome was assessed with the Thrombolysis in Myocardial Infarction (TIMI) scale.
RESULTS: Thirty-eight patients were included, with good outcome observed in 30 (79%) and poor outcome in 8 (21%) patients. Angiographic improvement based on TIMI scoring was seen in 24 (63%) patients, and no improvement was seen in 14 (37%). In 4 patients (11%), good outcome was obtained at 6 months despite no angiographic improvement on TIMI. No cases of intracranial rebleed or additional neurologic deficit following administration of abciximab were encountered.
CONCLUSION: In this small retrospective series, abciximab was safe and effective when used as a rescue agent for thromboembolic complications encountered during coiling of intracerebral aneurysms.
Endovascular treatment with detachable coils is, in many instances, the treatment of choice for intracranial aneurysms. Thromboembolism is a frequently reported complication, and one of the most difficult to treat. The reported incidence of hyperacute thromboembolic complications is seen to vary between 3% and 11% in previous case series.1–3 Results with angioplasty are poor, and the use of thrombolytics has been associated with an increased risk of rebleed. In one series, there was a 10% rebleed rate in patients receiving recombinant tissue plasminogen activator (rtPA) and mechanical clot disruption.4 In recent years, there has been an increasing interest in glycoprotein IIb/IIIa (GP IIb/IIIa) inhibitors to treat thromboembolic complications in this setting. Abciximab (ReoPro; Eli Lilly, Indianapolis, Ind) is the Fab fragment of the chimeric human-murine monoclonal antibody 7E3. Abciximab binds to the GP IIb/IIIa receptor of human platelets and inhibits platelet aggregation in the early stages of thrombus formation. It is, therefore, reasonable to administer abciximab as soon as possible after the onset of thromboembolism.
The therapeutic benefit of abciximab has been proved in coronary catheter intervention,5 lending evidence to its use in the treatment of thromboembolic complications encountered during intracranial aneurysm coiling. The current neuroradiology literature consists of several case reports6–9 and 3 case series comprising 13, 13, and 29 patients, respectively.10–12 These studies all report a favorable outcome when abciximab is used as a rescue agent in the treatment of thromboembolism, with a low incidence of rebleed. However when used in combination with thrombolytic drugs and mechanical clot disruption, a significant rebleed rate has been demonstrated. This suggests that risk of rebleed may be reduced when abciximab is used alone.
We report our experience using abciximab as a rescue agent for thromboembolic complications in 38 patients at a tertiary neurosciences center.
Materials and Methods
Patients
During a 6-year period between 2001 and 2007, 609 patients underwent endovascular coiling of intracranial aneurysms at our institution under 4 principal operators, who performed independently. A retrospective analysis of patients who received abciximab was performed. The corresponding patient case notes and angiographic images were reviewed by 2 neuroradiologists independently, resolving any points of contention by consensus. We collected the following data: patient demographics (age, sex), World Federation of Neurosurgeons (WFNS) grade at presentation (Table 1), acute or elective procedure, site of aneurysm, indication, route and regimen of abciximab, 6-month angiography findings, immediate and long-term outcome, and complications. In this period, 38 (6.2%) patients were identified in whom abciximab was administered intraprocedurally for thromboembolic complications witnessed angiographically during coil embolization.
WFNS grading
Procedure
All patients were treated under general anesthesia by 4 experienced neuroradiologists by using a standardized treatment approach. Systemic heparin was administered during coiling as a standard procedure, with the activated clotting time (ACT) being kept at least twice normal. This was not reversed if abciximab was given during the procedure. A 6F femoral vascular access sheath and closure device (Angio-Seal 6F; St. Jude Medical, St. Paul, Minn) was used in all cases.
Abciximab was administered as a bolus of 0.25 mg/kg (either intra-arterially or intravenously) in all cases in which thrombus development (occlusive and nonocclusive) was witnessed angiographically during the coiling procedure. This was then followed by a 12-hour intravenous infusion at 0.125 mcg/kg per minute. In cases in which thromboembolism occurred midprocedure, endovascular coiling was recommenced after the bolus administration of abciximab.
Outcome Measures
Outcome was classified as “good outcome,” with no significant clinical sequelae compared with the baseline neurologic status assessed on modified Rankin Scale (mRS) at 6 months, or “poor outcome,” with no resolution of a new clinical deficit that had developed since recovery on mRS at 6 months (Table 2). Angiographic resolution was scored according to the Thrombolysis in Myocardial Infarction (TIMI) criteria (Table 3), according to recognition of angiographic restoration of flow and arterial patency posttreatment.13
mRS score
TIMI Scale
Results
The 38 patients who received abciximab for the treatment of thromboembolic complications comprised 20 women and 18 men, with a mean age of 54 years (range, 29–81 years). Thirty-two (84%) patients underwent coiling for acutely ruptured intracranial aneurysms, with 6 (16%) undergoing elective procedures for unruptured aneurysms. The distribution of the treated aneurysms was 34 (89%) in the anterior circulation and 4 (11%) in the posterior circulation. In patients undergoing treatment of ruptured aneurysms, the preprocedural clinical grade according to the WFNS was 1 or 2 in 36 patients, with grades 4 and 5 in 2 patients.
A good outcome was observed in 30 patients (79%), and a poor outcome, in 8 (21%). All patients with good outcomes postprocedure showed no subsequent neurologic deterioration and had mRS scores of either 1 or 2 at 6-month follow-up. Angiographic improvement based on TIMI scoring was seen in 24 (63%) patients, with no improvement in 14 (37%). In 4 patients (11%), a good outcome was obtained at 6 months, despite no angiographic improvement on TIMI. Further assessment of these patients revealed good angiographic pial collateral supply to the affected arterial territory or, in the case of an involved A1 segment thrombus, good cross-flow from the contralateral side (Table 4).
Patient demographics and angiographic (TIMI), immediate clinical, and 6-month mRS outcome
Poor Outcomes
CT was performed within 5 days in patients with angiographic and corollary neurologic poor outcome. All patients in this group manifested a neurologic deficit and area of infarction corresponding to the vascular anatomic location of the angiographically witnessed thrombus. However, no radiologic evidence of rebleeding post-abciximab administration was identified on the postprocedural scans. Both patients (patients 8 and 17) classed as WFNS grades 4 and 5 preprocedure had poor outcomes; these patients were acknowledged to have had a poor pre- and periprocedural prognosis before treatment and the onset of thromboembolism. In 4 patients (11%), angiographic improvement on TIMI grading resulted in poor outcome. However, in 2 of these patients, the TIMI score improved from 0 to 1, and a further patient had a poor preprocedural WFNS grade (4, patient 17).
Illustrative Cases
Case 1: Basilar Artery Tip Aneurysm Coiling in a 68-Year-Old Man.
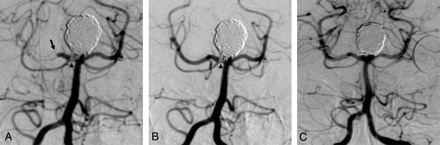
The patient presented with headache confirmed as subarachnoid hemorrhage (SAH) on CT. A basilar artery tip aneurysm with a narrow neck was demonstrated on diagnostic angiography. Endovascular coiling of the aneurysm was undertaken. On deployment of the last coil, a filling defect was noted at the origin of the right posterior cerebral artery (PCA) in association with a slight protrusion of a coil loop. Occlusive embolus was seen more distally within the same vessel (Fig 1A). An intra-arterial bolus of abciximab was administered stat via the 6F guide catheter. Angiography was performed 20 minutes after administration. Restoration of flow in the distal right PCA was achieved, but a small residual filling defect was seen at the origin of the vessel at the aneurysm neck (Fig 1B). Angiography 6 months later showed complete resolution of the filling defect and a patent right PCA (Fig 1C).
A, Thrombus formation at the origin (arrowhead) and distal total occlusion (arrow) of the right PCA in association with protrusion of a coil into the parent artery on near-completion angiography during coiling of a large basilar tip aneurysm. B, Angiogram 20-minutes post-intra-arterial abciximab demonstrates restored patency of the right PCA with a small residual amount of thrombus (arrowhead) at the aneurysm neck–parent artery interface. C, Angiogram 6 months after embolization demonstrates complete exclusion of the aneurysm and arterial patency with resolution of thrombus.
Case 2: Basilar Artery Aneurysm (left anterior inferior cerebellar artery origin) Coiling in a 29-Year-Old Woman.
The patient presented with a 24-hour history of neck stiffness and headache. There was no neurologic deficit at presentation (WFNS grade 1). CT and CT angiography demonstrated SAH and a basilar artery aneurysm arising from the origin of the left anterior inferior cerebellar artery. Angiography before the deployment of coils demonstrated the morphology of the aneurysm (Fig 2A). On deployment of the twelfth coil, a filling defect was observed at the neck of the aneurysm, with occlusion of the left PCA (Fig 2B). Abciximab was administered intravenously as a bolus dose stat with subsequent restoration of flow at 30 minutes after administration (Fig 2C). Two further coils were then deployed to complete the exclusion of the aneurysm. No neurologic deficit was evident postprocedure.
A, Precoiling angiogram demonstrates a large basilar artery aneurysm arising in close proximity to the origin of the left anterior inferior cerebellar artery. B, Mid coil-embolization of the aneurysm with a small thrombus causing a filling defect in the parent vessel (arrowhead) and near-total occlusion of the left PCA (arrow). C, Approximately 30 minutes following the administration of an intravenous bolus of abciximab, there is demonstrable restoration of flow within the left PCA.
Discussion
Thromboembolic complications are among the most common encountered during intracerebral aneurysm coiling and one of the most challenging to treat. These complications are reported in 3%–11% of patients during the procedure, with permanent associated neurologic deficit rates quoted at 1%–5%.1–3
There has been increasing interest and debate about the treatment of hyperacute intraprocedural thromboembolic complications. Fibrinolytic agents such as rtPA or urokinase have been used in this setting with varying degrees of outcome. Cronqvist et al4 reported using intra-arterial urokinase in 19 patients undergoing endovascular aneurysm coiling; in 3 of 6 patients with ruptured aneurysms, there was extensive intracranial hemorrhage with resulting marked neurologic impairment. This is an important consideration, especially in patients undergoing the procedure to treat an acutely ruptured aneurysm.
Platelet functional activation is the predominant process in the hyperacute stages of clot formation, sometimes referred to as “white clots.” In contrast, “red clots” are fibrin-rich and occur in the latter stages of clot formation. An ideal agent to treat hyperacute clots would, therefore, be one that rapidly inhibits platelet function. Abciximab and other glycoprotein IIb/IIIa inhibitors have received particular attention due to their apparent safe and effective use in this setting as well as their relatively short half-lives. Abciximab is a chimeric human/mouse monoclonal antibody, is a potent inhibitor of the platelet GP IIb/IIIa receptor, and prevents platelets from binding to fibrinogen and platelet aggregation. This action, by implication, confers an advantage to GPIIb/IIIa inhibitors over fibrinolytics in the dissolution of hyperacute thrombus. Abciximab has a dual-phase half-life, the first lasting 10 minutes and a second lasting 30 minutes. The relatively short half-life has important advantages, particularly in the setting of the development of a thromboembolic complication during endovascular coiling of an acutely ruptured aneurysm midprocedure. In such circumstances, continued packing of the aneurysm sac may be likely in order to exclude fully or protect the neck of the aneurysm once thrombus dissolution has been achieved, perpetuating the risk of an intraprocedural rupture. However, postadministration, 80% of the GPIIb/IIIa receptors are blocked and platelet function is inhibited for approximately 48 hours, with a low-level blockade observed for up to 14 days. The antiplatelet function of abciximab cannot be acutely reversed, but platelet transfusions can be administered in case of hemorrhage.
Most evidence for the use abciximab to prevent clot formation has been carried out with trials in coronary artery intervention and the treatment of acute coronary syndromes. The EPILOG (evaluation of percutaneous transluminal cornary angioplasty to improve long-term outcome by GPIIb/IIIa receptor blockade) investigators concluded that abciximab, together with low-dose heparin, markedly reduces the risk of acute ischemic complications in patients undergoing percutaneous coronary revascularization, without increasing the risk of hemorrhage.5 Currently, these are the only on-label indications for the use of abciximab.
Our use of abciximab and that of others in the neuroendovascular setting are largely based on the cardiology experience and selected small case series. There have been several case reports and a few case series that have reported the apparent safe and effective use of abciximab as a rescue agent in the neurovascular setting. The largest series is that reported by Velat et al12 in 29 patients (26 undergoing intracerebral artery coiling) to whom abciximab was administered. In our study, abciximab was given when the thromboembolism was witnessed angiographically, regardless of whether satisfactory aneurysm exclusion was achieved. Some authors recommend that the aneurysm be occluded before the administration of abciximab.3,7,8,12 However, other series detail evidence contrary to these earlier recommendations. Aviv et al10 observed no increased hemorrhage, with partially occluded or unoccluded aneurysms. Our experience further supports this observation in that no delay was imposed after the thromboembolism was witnessed before abciximab was administered, irrespective of the stage of aneurysm packing. Furthermore, patients in our series had coils deployed in subtotally packed or adequately excluded aneurysms following abciximab administration.
No other agents (other than heparin) were used in any of our patients because the use of other agents and techniques for treatment of thrombus forming during endovascular treatment of aneurysms (thrombolytics or mechanical clot disruption) has been associated with higher bleeding rates.
None of our patients had rebleed complications, thereby strengthening the argument for immediate abciximab administration for thromboembolic complications. One of our patients did, however, experience a mild non-life-threatening upper gastrointestinal bleed 24 hours post-abciximab administration. Similar series have, however, reported select cases of rebleeding. Aviv et al10 reported a case of rebleed and mortality, in which further coil placement occurred postadministration of abciximab. The drug manufacturers recommend reducing the heparin effect with abciximab delivery. The Evaluation of Platelet IIb/IIIa Inhibition for Prevention of Ischemic Complications (EPIC) trial demonstrated a threefold increase in bleeding complications with combined drugs when 10,000–12,000 U of heparin was given intravenously.14 In the EPILOG trial using 70 U of heparin per kilogram, there was a lower bleeding complication rate than that in the EPIC trial. Also, an ACT of 225 seconds with combined drugs (abciximab and heparin) was equivalent to an ACT of 35–400 seconds with heparin alone. ACT was maintained at 200–300 seconds, and a single bleeding complication was reported.
In all of our patients, heparin was given intravenously prophylactically (ACT maintained between 200 and 300 seconds) during the procedure, which was not reversed after administration of abciximab. There is a consensus approach to stopping heparin in this setting based on previous series. Velat et al12 stopped heparin following administration of abciximab (but did not reverse anticoagulation) in all their patients and did have 3 patients with rebleed. Song et al3 stopped heparin in all 7 of their reported patients, and only reversed this with protamine in 1 patient.
A factor that seems varied in the literature is the route of administration of the initial abciximab bolus. In the early cases of our series, we administered this via an intravenous bolus according to weight (0.25 mg/kg). There is some evidence, reported by Mounayer et al,11 suggesting that a selective arterial initial low-dose bolus, followed by intravenous infusion, offers a safe and effective outcome. This study, along with others,12 and our own experience suggest that administration through a microcatheter selectively placed in the vicinity of the thromboembolus is a safe and effective route.
Conclusion
In this small retrospective series of 38 patients, abciximab was safe and effective when used for thromboembolic complications encountered during coiling of intracerebral aneurysms. Our institutional experience is similar to that of several smaller published case series and further supports the use of abciximab as a rescue agent in this setting.
Footnotes
Paper previously presented at: Annual Meeting of the Radiological Society of North America, November 30, 2006; Chicago, Ill.
References
- Received January 7, 2008.
- Accepted after revision June 24, 2008.
- Copyright © American Society of Neuroradiology