Abstract
Summary: Two cases of pontine infarct with Wallerian degeneration (WD) of the pontocerebellar fibers are described. WD of pontocerebellar fibers, seen bilaterally along the transverse pontine fibers, is more visible in the middle cerebellar peduncles and extends into the white matter of the cerebellar hemispheres. Understanding the anatomy of the white matter and the temporal evolution of this degeneration is essential in identifying WD.
Wallerian degeneration (WD) is the process of progressive demyelination and disintegration of the distal axonal segment following the transection of the axon or damage to the neuron. Although this term originally referred to lesions of peripheral nerves, today it can also refer to the CNS when the degeneration affects a fiber bundle or tract through the same mechanism.
The most commonly recognizable cause of WD is cerebral infarction. WD can also result from a variety of conditions including hemorrhage, trauma, necrosis, and focal demyelination (1, 2).
MR imaging may depict WD when it involves a sufficiently large bundle of fibers. The most common observations regard the corticospinal tract. If attention is paid to the course of fibers that may be affected by a certain lesion, however, degeneration of fibers crossing the corpus callosum, fibers of the optic radiations, fornices, and cerebellar peduncles may be recognized (3–5). Knowledge of the course of fibers is essential in recognizing WD. An excellent review of the lesions that may affect the middle cerebellar peduncles (MCPs) was recently published (6). This review by Okamoto et al quotes an article that, to our knowledge, is the only current report of WD of pontocerebellar fibers caused by an acute vascular lesion in the pons (7). Very few other reports illustrate or mention MCP abnormalities in WD (8, 9). The course of the pontocerebellar fibers in humans is still not widely known. To understand how pontine infarcts may affect these fibers, Schmahmann et al recently carried out a very interesting study in monkeys (10).
Here we describe two cases in which a focal ischemic pontine lesion caused signal intensity abnormalities, demonstrated by MR imaging, along the course of the fibers from the pons to the MCPs in all planes.
Case Reports
Case 1
A 77-year-old man, a smoker without other cardiovascular risk factors, was hospitalized at another institution, presenting with speech difficulties, right hemiparesis evolving into progressive deterioration with left gaze paralysis, peripheral facial palsy, and drowsiness (Glasgow coma scale 12). CT and MR imaging were performed and demonstrated a left pontine infarct and minimal signal intensity abnormalities in the white matter of the centra semiovalia. He was discharged with right hemiparesis. He was admitted to our institution 7 months later because of transient worsening of right limb weakness. Neurologic examination showed severe right spastic hemiparesis more evident in the upper limb. Vertebral and carotid artery ultrasonography (US) findings were normal for his age. MR imaging showed a hyperintense lesion on T2-weighted images on the left side of the pons, compatible with an ischemic lesion in the territory of the left perforating branches of the basilar artery. Symmetrical, moderately hyperintense lesions were also visible in both MCPs, fading into the white matter mainly in the upper part of the cerebellar hemispheres. MR angiography (MRA) showed irregularities and stenosis of the left vertebral artery at the confluence into the basilar artery, while the right vertebral artery seemed to end in the posterior inferior cerebellar artery (PICA): the segment between the origin of the PICA and the basilar artery was not visible. The patient was discharged with antithrombotic treatment (Warfarin).
Case 2
A 61-year-old man, with a genetic mutation of factor V Leiden and a history of arterial hypertension and noninsulin-dependent diabetes mellitus, was admitted to our institution for clinical and diagnostic evaluation of an ischemic stroke. His neurologic symptoms had begun 7 months earlier with transient diplopia, disarthria, and left-sided weakness followed by good clinical recovery. On that occasion, the patient was submitted to a first MR imaging examination, which showed a small pontine infarct. Five months later, he presented acute postural vertigo and weakness of the left upper and lower limbs and had a second MR imaging study. Because of persistence of these fluctuating symptoms, the patient was admitted to our institution, presenting mild left pyramidal hemisyndrome at neurologic examination. Electrocardiography and vertebral and carotid US studies showed normal findings for his age. A transthoracic echocardiogram showed slight mitral regurgitation. MR imaging showed a T2 hyperintense linear image on the right side of the pons close to the midline, consistent with an infarction in the territory of the right paramedian perforating branches of the basilar artery (Fig 1A). Diffuse T2 hyperintense and T1 hypointense abnormalities were also recognizable along the pontine transverse fibers and in both MCPs, extending to the white matter of the upper part of the cerebellar emispheres (Fig 1B–D). Posterior periventricular T2 hyperintense abnormal areas were recognizable in the cerebral hemispheres consistent with dilated perivascular spaces and chronic ischemic changes. No enhancement was present after administration of contrast material. MRA showed slight irregularities of the walls of both carotid siphons and normal basilar artery. Review of previous studies showed that the pontine infarct was present on both MR images, while the MCP abnormalities were absent on the first study, performed at the onset of symptoms, but became visible on the second one. The patient was discharged with Warfarin therapy.
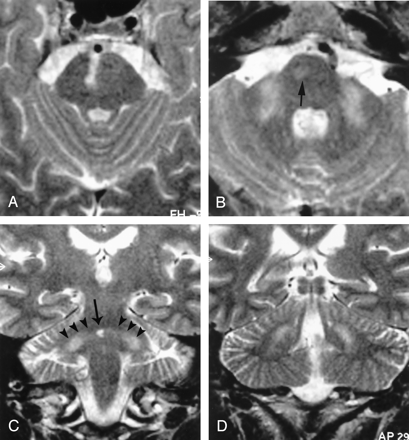
Patient 2.
A and B, Axial T2-weighted nonconsecutive sections, 4 mm thick. On the cranial section (A) only the right paramedian pontine infarct is visible. On the caudal section (B) hyperintensities of MCPs are visible. Faint right pontine hyperintensity represents WD along the corticospinal tract (arrow).
C, Coronal T2-weighted section shows the right paramedian infarct (arrow) and a slighter hyperintensity of the degenerated transverse pontine fibers and MCPs (arrowheads). Note that these fibers are caudally oriented from the midline to the MCP.
D, WD extends posteriorly in the cerebellar hemispheres, mostly in the upper part.
Thirteen months after his first admission to our institution, he was readmitted because of bilateral visual impairment and transient left diplopia. Neurologic examination showed mild deficit of the left sixth cranial nerve, and left motor hemisyndrome, which was unchanged with respect to the first admission. MR imaging showed that the ischemic pontine lesion was also unchanged. The T2 hyperintense lesion recognizable in pontine transverse fibers and in both MCPs was less extended and less evident. The remaining MR imaging findings and the carotid and vertebral US findings were unchanged.
Discussion
WD is a process that develops through different stages. The first stage is characterized by the physical disintegration of the axons and myelin sheaths with little chemical changes. The process is slow in the CNS, much slower than in the peripheral nervous system, and it is unusual to find positive Marchi material (lipids secondary to degradation of the myelin sheath) before the 20th day after the lesion or insult causing WD (2).
The second stage is characterized by the rapid destruction of the myelin fragments observed in the first stage. In humans, within 3 months most of the myelin has broken down into simple lipids and neutral fats, cleared by phagocytosis (11). In the third stage, the myelin sheath has almost disappeared, and gliosis occupies the area of the degenerated axons and myelin sheaths.
These histologic and metabolic features are correlated to specific findings at MR imaging. In stage 1, no signal intensity abnormalities are usually recognizable. From 20 days to 2–4 months after stroke (stage 2), the tissue becomes more hydrophobic because of the myelin-protein breakdown: the high lipid-protein ratio results in hypointense signal intensity in proton density–and T2-weighted images. Hypointense signal changes are usually recognizable earlier in proton density–versus T2-weighted images because of the different conspicuity of the low signal intensity of the degenerated fibers versus the background. Stage 3 results from subsequent myelin lipid breakdown, gliosis, and changes in water content and structure; the tissue becomes hydrophilic and there is hyperintense signal on T2-weighted and FLAIR images and hypointense signal on T1-weighted images. After several years, the end stage (stage 4) is characterized by volume loss from atrophy, which, for instance, may be recognized in the brain stem as unilateral shrinkage following WD of the corticospinal tract (1). These temporal relationships between the MR imaging findings and the stages of WD are usually recognized; however, there is a great variability, and signal intensity abnormalities may be visible many years after stroke.
In our second case, the MR imaging performed a few days after the occurrence of pontine infarct did not show any T2 hyperintense signal abnormality of the MCPs. On the second MR imaging examination 5 months after stroke, T2 hyperintense signal intensity abnormality along the pontine transverse fibers and in both MCPs was recognizable. MR imaging performed 2 months later was unchanged, but the MR imaging examination performed 13 months later (20 months after the stroke) showed a decrease in the MCPs T2 hyperintense signal abnormalities. This evolution of signal intensity changes is consistent with the different stages described above.
The MCPs are a massive bundle of fibers connecting the basal portion of the pons with the cerebellum. In the base of the pons, scattered between the descending corticospinal, corticopontine, and corticobulbar fibers are the pontine nuclei that give origin to the horizontally oriented pontocerebellar fibers. The pontine nuclei receive input from the cerebral cortex via the corticopontine tract and project almost exclusively to the contralateral cerebellum via the transverse pontine fibers and MCPs, which constitute the pontocerebellar tract. The cortico-ponto-cerebellar organization has been studied in monkeys. Areas 4 and 6 are the major source of input from the cerebral to the cerebellar cortex, but primary sensory areas 3, 1, 2, parietal areas 5 and 7, and superior temporal areas also contribute. Each area projects to a slab at the level of the pontine nuclei and to a given area of the cerebellar cortex. Within a given cerebral cortical area, a small point of cortex may project to several points in a pontine slab and on the cerebellar surface. This has been called “fractured somatotopy,” which is a typical feature of the mossy fiber system. Pontocerebellar fibers project to every lobule of the cerebellar hemispheres and in the vermis, mainly to the declive, folium, tuber, and uvula (12, 13). The cortico-ponto-cerebellar pathway is quantitatively the most important route by which the cerebral cortex can influence the contralateral cerebellar cortex in the preparation, initiation, and execution of movement.
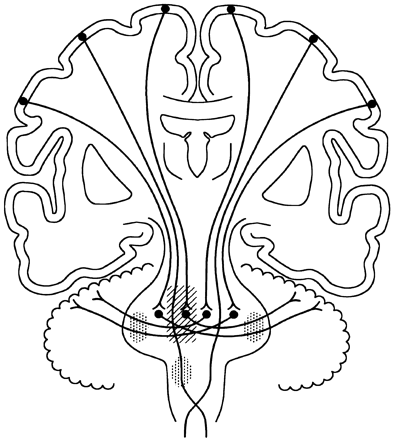
The signal intensity abnormalities due to WD in MCPs were bilateral in both of our cases and in those reported in two other previous articles, because of the crossed distribution of the pontocerebellar fibers (7, 9). Bilateral T2 abnormalities of the MCPs are also mentioned in cases of WD of the pontocerebellar fibers due to central pontine myelinolysis (8). Even a narrow strip of a paramedian infarct close to the raphe, as seen in our case 2, damages the medial part of the ipsilateral pontine nuclei and the axons of the more lateral neurons, which will have to cross the midline to reach the contralateral MCPs and cerebellar hemisphere; the lesion also damages the axons originating from the contralateral pontine nuclei that have crossed the midline and course through the pons to reach the ipsilateral MCP and cerebellar hemisphere (7) (Fig 2). Coronal sections depict the continuity of the degenerated fibers better than the axial sections (Fig 1).
Schematic drawing illustrating the corticopontine tracts and pontocerebellar fibers with their synapses in the pontine nuclei. The corticospinal tracts are also illustrated. The right pontine lesion (obliquely lined area) damages the local pontine neurons and the fibers originating more laterally on the right side, which will have to cross the midline, and all the fibers coming from the left pontine nuclei. The WD is best seen in the MCPs (dotted areas). WD is also present along the right corticospinal tract.
In addition to pontine infarcts or hemorrhages, or other acute lesions that may cause WD, several pathologic conditions may affect the MCPs and cause symmetrical and bilateral hyperintensity on T2-weighted images (6). In these cases, however, other mechanisms, like chronic neuronal degeneration, should be considered. T2 hyperintensity in the MCPs is a well-known MR imaging finding in patients with neurodegenerative diseases, such as multisystem atrophy with predominant cerebellar signs (MSA-C), in which it is associated with the typical “cross-sign” (14, 15). In our experience, MSA-C is the most common condition in which signal intensity abnormalities of MCPs are present. MCP abnormalities may also be seen in spinocerebellar ataxias types 2 and 3, Wilson disease (6), and fragile X premutation (16).
Conclusion
The basic understanding required to diagnose WD in the brain is a detailed knowledge of the course of the most important projection and association fibers. In a patient with the appropriate clinical presentation, the observation of a pontine lesion with symmetrical signal intensity abnormalities of the MCPs makes it possible to recognize WD of the pontocerebellar fibers. WD should not be mistaken for a primary, independent lesion. Coronal sections, by demonstrating the continuity of the signal intensity abnormalities, facilitate the recognition of the degenerated transverse pontine fibers. Diffusion tensor imaging may further enhance the possibility of diagnosing WD.
Footnotes
Dr. Tiziana De Simone and Dr. Caroline Regna-Gladin contributed equally to the preparation of this manuscript.
References
- Received April 16, 2004.
- Accepted after revision July 30, 2004.
- Copyright © American Society of Neuroradiology