Abstract
BACKGROUND AND PURPOSE: Cortical lesions constitute a substantial part of the total lesion load in multiple sclerosis (MS) brain. They have been related to neuropsychological deficits, epilepsy, and depression. However, the proportion of purely cortical lesions visible on MR images is unknown. The aim of this study was to determine the proportion of intracortical and mixed gray matter (GM)-white matter (WM) lesions that can be visualized with postmortem MR imaging.
METHODS: We studied 49 brain samples from nine cases of chronic MS. Tissue sections were matched to dual-echo T2-weighted spin-echo (T2SE) MR images. MS lesions were identified by means of myelin basic protein immunostaining, and lesions were classified as intracortical, mixed GM-WM, deep GM, or WM. Investigators blinded to the histopathologic results scored postmortem T2SE and 3D fluid-attenuated inversion recovery (FLAIR) images.
RESULTS: Immunohistochemistry confirmed 70 WM, eight deep GM, 27 mixed GM-WM, and 63 purely cortical lesions. T2SE images depicted only 3% of the intracortical lesions, and 3D FLAIR imaging showed 5%. Mixed GM-WM lesions were most frequently detectable on T2SE and 3D FLAIR images (22% and 41%, respectively). T2SE imaging showed 13% of deep GM lesions versus 38% on 3D FLAIR. T2SE images depicted 63% of the WM lesions, whereas 3D FLAIR images depicted 71%. Even after side-by-side review of the MR imaging and histopathologic results, many of the intracortical lesions could not be identified retrospectively.
CONCLUSION: In contrast to WM lesions and mixed GM-WM lesions, intracortical lesions remain largely undetected with current MR imaging resolution.
Multiple sclerosis (MS) is an inflammatory, demyelinating disease that usually affects young adults and leads to chronic disability. Although MS has been regarded as a disorder predominantly affecting the (periventricular) white matter (WM), involvement of the gray matter (GM) was already acknowledged in early pathologic studies (1–3). Renewed interest in GM disease has revealed that the prevalence of cortical lesions is high (4–7). Histopathologic depiction of the intracortical MS lesions has improved with the use of myelin protein immunohistochemistry, and purely intracortical lesions have been found to account for most of the total cortical demyelination (5, 6).
Different classification systems for cortical MS lesions have been proposed, among them the system of Bö et al (6), which is based on the anatomic observations by Dawson (1). This system distinguishes mixed GM-WM lesions (type I cortical lesions) from purely intracortical lesions (types II-IV). Type II lesions are small intracortical lesions, type III lesions are larger lesions extending from the pia downwards without reaching the subcortical WM, and type IV lesions affect the entire width of the cortex from pial surface to the WM. Type III lesions are most frequently seen (5, 6) and can extend over several gyri, in certain cases leading to a general cortical subpial demyelination (7).
With MR imaging, it is difficult to determine whether lesions are intracortical, subcortical, or combined cortical-subcortical, which explains the introduction of the term juxtacortical lesion. Juxtacortical lesions are important in a diagnostic setting (8), since the U fibers are often spared by hypoxic-ischemic small-vessel disease, but juxtacortical lesions are frequently found in MS. (Juxta)cortical lesion load and atrophy, as determined by MR imaging, have been correlated to physical disability, epilepsy, cognitive impairment, and depression (9–16).
Studying the effect of cortical demyelination on the physical and neuropsychological status of the MS patient is hindered by an apparent low sensitivity of MR imaging for GM lesions (4, 17). It is not known what the exact sensitivity of MR imaging for the intracortical versus mixed GM-WM and deep GM lesions is.
In this study, MS brain samples containing cortical GM were collected, and histopathologic sections of these samples were compared with postmortem MR images obtained at autopsy. The purpose of the study was to determine the proportion of mixed GM-WM (type I) and intracortical (type II-IV) lesions visible on postmortem MR imaging.
Methods
Patients and Autopsy
Data from nine patients with MS were studied after rapid autopsy (mean postmortem delay, 7 hours 50 minutes). Forty-nine brain tissue samples were obtained. Table 1 shows the patients’ characteristics. Permission for performing autopsies, for the use of tissue, and for access to medical records for research purposes was granted by our local ethical review board. Tissue sampling and autopsy procedures have been described earlier (18, 19). Briefly, 10-mm-thick coronal brain sections were cut, and four to five sections were subjected to MR imaging. WM abnormalities visible on postmortem T2-weighted spin-echo (T2SE) imaging and areas of GM were sampled.
Clinical and pathologic data of the patients with MS
MR Imaging
Standard dual-echo T2SE images (TR/TE/NEX, 2755/90 and 45/2) and 3D FLAIR images (TR/TE/TI/NEX, 6500/120/2200/1) of selected 10-mm brain sections were acquired by using a 1.5T machine (Vision; Siemens Medical Systems, Erlangen, Germany). In-plane resolution was 0.64 mm2 for both T2SE imaging (section thickness, 5.0 mm), and 3D FLAIR imaging (section thickness, 1.25 mm; eight sections per slab).
Histology
Serial 5-μm-thick sections fixed in 10% formalin and embedded in paraffin were mounted onto glass slides (Superfrost; Menzel-gläser, Braunschweig, Germany) and dried overnight at 37°C. Sections were deparaffinated in a series of xylene, 100% alcohol (ethanol), 96% alcohol, and 70% alcohol and water. Endogenous peroxidase activity was blocked by incubating the sections in methanol with 0.3% H2O2. Tissue sections were microwaved for 10 minutes in a 10 mmol/L citrate buffer (pH 6.0) for antigen retrieval. After the sections cooled sufficiently, they were rinsed. All washes were carried out for 30 minutes with 0.01 mol/L phosphate-buffered saline (PBS, pH 7.4), and antibodies were diluted in PBS containing 0.1% bovine serum albumin (BSA). To prevent nonspecific binding, sections were preincubated with PBS containing 5% BSA for 10 minutes at room temperature.
Primary antibodies (myelin basic protein [MBP], Boehringer Mannheim, Mannheim, Germany) were diluted 1:100 in PBS-BSA and incubated for 1 hour at room temperature. After washing, immunolabeling with primary antibodies was detected with biotinylated rabbit anti-mouse (1:500) for 30 minutes at room temperature and avidin-biotin-peroxidase complexes (sABC-HRP 1:200; Dako, Glostrup, Denmark) for 60 minutes at room temperature.
Peroxidase activity was demonstrated with 0.5 mg/mL 3,3′ diaminobenzidine tetrahydrochloride (DAB; Sigma, St. Louis, MO) in PBS containing 0.03% H2O2 for 5 minutes, which led to a brown reaction product. Sections were counterstained with hematoxylin and mounted (Depex, BDH; Poole, UK).
Matching
Samples were cut from the imaged plane (i.e., the middle of the 10-mm-thick brain section). MBP-stained tissue sections were then carefully matched to the postmortem T2SE images by using cortical anatomy, ependymal lining, and WM lesions as landmarks. Matching was performed according to a protocol previously described (18, 19).
Analysis
Numbers of mixed GM-WM (type I), intracortical (type II–IV), deep GM, and WM lesions were scored on T2SE and 3D FLAIR images. Lesions were defined as clearly circumscribed areas of abnormal signal intensity on the MR image; confluent abnormalities were scored as one lesion (same for histopathology). The reader’s (J.J.G.G.) scoring was blinded to histopathologic results and reviewed by an experienced neuroradiologist (F.B.). The pathology reader scored lesion numbers and lesion types (type I–IV, deep GM or WM) by using the MBP-stained tissue sections and was blinded to the MR data.
Lesion numbers scored on the T2SE and 3D FLAIR images were compared with each other and with the lesion numbers obtained from the matched histopathologic areas (the criterion standard). The amounts of lesions visible on T2SE and 3D FLAIR images were expressed as percentages of the amount of lesions scored histopathologically.
After the blinded scoring of the MR images was done, the precise localizations and types of all lesions were revealed to the MR readers for a retrospective assessment.
Results
In general, MR images were well matched to the histopathologic sections (Fig 1). A total of 168 lesions were identified in the 49 tissue samples by MBP immunohistochemistry (Fig 2, Table 2): 98 GM lesions (27 type I lesions, 12 type II, 41 type III, 10 type IV, eight deep GM) and 70 WM lesions. Thus, almost equal numbers of GM lesions and WM lesions were found.
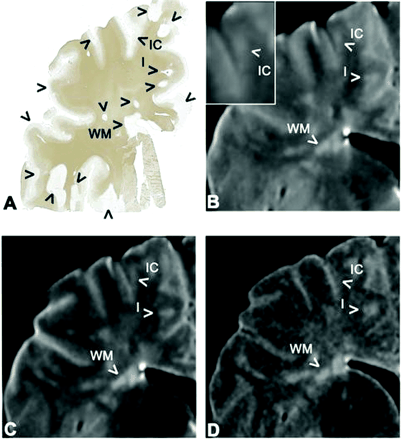
Example of a tissue sample and the matching area on postmortem MR images. WM lesions (WM), as well as type I lesions (I, mixed GM-WM), can be seen with relative ease on the different MR images. Intracortical lesions (IC) are difficult to detect and define, even in retrospect.
A, Photomicrograph (MBP immunohistochemical stain) reveals lesions (arrowheads) in the WM and cortical GM.
B, Short-echo T2-weighted SE image. Insert, a higher magnification of the intracortical lesion.
C, Long-echo T2-weighted SE image.
D, 3D FLAIR image.
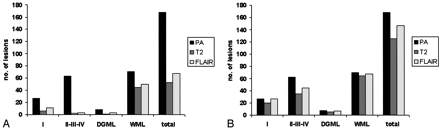
Lesion scores. I, type I cortical lesions; DGML indicates deep GM lesions; WML, WM lesions; total, total of all lesion categories; PA, lesions detected histopathologically; T2, lesions detected on postmortem T2SE imaging; and FLAIR, lesions detected on postmortem 3D FLAIR imaging.
A, Blinded.
B, After unblinding, numbers of detected lesions in all categories increased (retrospective scoring). However, a significant proportion of intracortical lesions remained undetectable.
Lesions found on T2SE and 3D FLAIR and comparison with histopathology
Blinded T2SE analysis revealed 53 lesions (32% of the histologically detected lesions), of which 44 were WM lesions (63% sensitivity). Only two intracortical lesions were detected (3%). 3D FLAIR imaging revealed 67 lesions (40%), of which three were intracortical lesions (5% of the criterion standard). Considering the large number of WM lesions detected with both T2SE imaging (44 lesions, 63% of the total) and 3D FLAIR imaging (50 lesions, 71% of the total), differences between the techniques were rather small in this category. One deep GM lesion was observed with T2SE (13%), while 3D FLAIR depicted three (38%). Type I lesions were best detected with 3D FLAIR (11 lesions, 41% sensitivity); T2SE showed six type I lesions (22% sensitivity). Most T2SE-visible lesions were also observed on 3D FLAIR images, and vice versa. After the classification and precise localization of all lesions were revealed to the primary investigator, T2SE and 3D FLAIR images were reassessed. Higher numbers of lesions were recognized in retrospect (Fig 2, Table 2). Nevertheless, 44% and 29% of intracortical lesions remained invisible on T2SE and 3D FLAIR, respectively. Interestingly, all type I lesions could be retrospectively detected with 3D FLAIR, versus 74%, with T2SE study. Visibility of deep GM lesions on T2SE and 3D FLAIR images was also better retrospectively; the images respectively showed 63% and 88% of the lesions identified on histologic analysis.
Discussion
Previous studies have emphasized the abundance of lesions in the cerebral cortex and deep GM in MS (2–4). With myelin immunohistochemistry, it has been found that the most common lesion types are intracortical lesions, which may account for more than 85% of total cortical demyelinated area (7). Although we did not systematically assess the proportion of lesions, most were found in the GM. The numbers and distribution of cortical lesions in this investigation were comparable to those of earlier studies (5, 7). Our classification system for cortical lesions allowed for detailed definition concerning the anatomic localization of lesions within or in direct proximity to the cortex. This distinction is important because different types of cortical lesions behave differently in terms of their histopathologic characteristics. For example, purely intracortical lesions (types II–IV), which are difficult to visualize with MR imaging, do not show substantial T lymphocyte infiltration. On the other hand, mixed GM-WM lesions, which are better detectable with MR imaging, do display a clear inflammatory reaction (6). This means that the group of cortical lesions is heterogeneous and that the sensitivity of MR imaging for different cortical lesion types may vary depending on lesion type.
The detection of cortical lesions is hampered by the fact that little myelin is present in the cortex; this causes T2 relaxation times of cortex to be higher than those of WM. As a result, only small increases in T2 relaxation times occur in cortical lesions, in contrast to WM lesions, which show a marked increase of T2 with respect to surrounding WM. Furthermore, partial volume effects with CSF in sulci may impede lesion detection on MR imaging. This results in poor intracortical lesion scores, even when performed by experienced neuroradiologic readers. The detection of cortical lesions with standard myelin histochemical stains such as Luxol fast blue is also difficult.
Because an experienced neuropathology reader reviewed the histopathologic features of MR-visible lesions, it could be ascertained that the cortical lesions were not due to hypoxic-ischemic causes.
Previous groups relating the histopathologic results to postmortem MR imaging findings have tried oil red O, galactocerebroside (17), and Heidenhain myelin stain (4) to improve the detection of intracortical lesions. MBP immunohistochemical results may be more accurate markers for intracortical myelin (20). This method has proved to be sensitive to intracortical demyelination and allows for accurate classification of lesion type (5, 7). Another factor that may limit the visibility of cortical lesions on MR imaging (and standard histochemistry) is the absence of an inflammatory reaction in intracortical lesions and even in the cortical part of type I lesions.
Results of many in vivo studies have suggested the use of FLAIR to improve cortical lesion detection (9, 10, 21–25). In this study, however, the gain with 3D FLAIR imaging, compared with T2SE imaging, was limited, although the section thickness of T2SE is larger and the detection of small lesions could therefore be expected to decrease through signal intensity averaging. The 3D FLAIR sequence that we used is routinely applied in clinical practice and normally shows good CSF suppression, but it was found to show suboptimal fluid suppression on postmortem MR imaging. The most likely explanation is that the fluid surrounding the brain sections does not consist of pure CSF, but rather, it contains varying amounts of blood and proteins, leading to relaxation properties that need a different TI for suppression. The fact that postmortem T2SE and 3D FLAIR images had different section thicknesses (5.0 and 1.25 mm, respectively), may be a source for the different sensitivities of the two techniques. This variation was done to compare a typical standard resolution and a higher resolution; nevertheless, both T2SE and 3D FLAIR images showed almost equally low numbers of intracortical lesions. Besides histopathologic studies revealing substantial cortical lesion loads (2–4, 6, 7, 17), quantitative MR studies have shown several abnormalities in the GM of patients with MS. Changes in metabolite concentrations in MS cortical GM were found in studies using MR spectroscopy (26, 27), even in early stages of the disease (28). Moreover, magnetization transfer and diffusion tensor or mean diffusivity studies revealed notable abnormalities in MS cortical GM (29–34). However, the extent to which these changes reflect cortical lesions or diffuse nonlesion disease is unknown.
The poor intracortical lesion detection on postmortem MR imaging, as shown in this study, has important implications for the sampling of these lesions, impeding further studies with respect to specific histopathologic characteristics of cortical lesions. Intracortical lesions did not show inflammation, and other features, like edema, were also not observed in the lesions found histopathologically. Further investigation into whether any other histopathologic characteristics may contribute to visibility on MR imaging would be interesting. Unfortunately, the small number of cortical lesions detected on MR imaging in this study makes any phenotypic comparisons difficult. However, judging from the fact that our (unblinded) retrospective analysis of the MR images showed many relatively small changes in signal intensity in the areas of cortical lesions, the imaging of brain sections at higher resolution (e.g., at a field strength of 3T) or by using techniques such as 3D double-inversion recovery (35–37) could well improve postmortem cortical lesion detection.
In vivo and postmortem T2SE studies are generally of comparable quality, and equal numbers of lesions are detected, indicating that our results may also apply to the in vivo situation. Intracortical MS disease is thus unlikely to be detectable in vivo at 1.5T by using routine imaging techniques. This situation is unfortunate because the detection of cortical lesions may be relevant for patients with MS who develop cognitive symptoms early in the course of their disease or for MS patients with epilepsy. Purely cortical lesions could also lead to motor or sensory signs and symptoms, thereby contributing to the clinicoradiologic dissociation observed in MS.
Conclusion
WM and mixed GM-WM (type I) lesions are relatively easily detected on postmortem T2SE and 3D FLAIR imaging. However, intracortical lesions (type II–IV), as defined by MBP histopathologic reports, remain largely undetected on MR imaging. Even on retrospective analysis, many of the intracortical lesions could not be seen. This information may have important consequences for postmortem sampling of cortical abnormalities and the in vivo investigation of clinical and neuropsychological abnormalities in patients with MS.
Acknowledgments
The authors would like to thank the Netherlands Brain Bank (coordinator, Dr Rivka Ravid) for providing the brain tissue; Drs Wouter Kamphorst and Paul van der Valk for their supervision at autopsy and their description of the specimens; Lisette Montagne and Elise van Haastert for their excellent technical support; Dr Hugo Vrenken for help with the acquisition of the MR data; and Dr Bernard Uitdehaag for the critical review of this paper.
Footnotes
MS Research Foundation, Voorschoten, the Netherlands (grant no. 00-427 MS).
Presented at the 19th Meeting of the European Committee of Treatment and Research In Multiple Sclerosis, Milan, Italy, September 17–20, 2003.
References
- Received May 5, 2004.
- Accepted after revision June 15, 2004.
- Copyright © American Society of Neuroradiology