Abstract
BACKGROUND AND PURPOSE: Therapeutic internal carotid artery (ICA) occlusion for symptomatic intracavernous artery aneurysms can result in ischemic infarction despite normal clinical balloon test occlusion (BTO). We evaluated outcomes in patients with symptomatic cavernous sinus aneurysms in whom clinical BTO was normal, who underwent carotid occlusion with selective bypass surgery guided by physiologic BTO using quantitative cerebral blood flow (CBF) analysis by means of stable xenon-enhanced CT.
METHODS: After a normal clinical BTO, 26 consecutive patients with symptomatic cavernous sinus aneurysms underwent a baseline xenon-enhanced CT CBF analysis followed by a second CBF analysis, during which repeat BTO was performed. Patients with a decrease in cortical CBF to below 30 mL/100 g/min were considered moderate risk and those with greater than 30 mL/100 g/min were low risk for developing postocclusion ischemic infarction. Moderate-risk patients underwent cerebral revascularization followed by proximal carotid occlusion. Low-risk patients underwent carotid occlusion alone. Patients were clinically followed up for at least 3 months after carotid occlusion. All patients underwent head CT at least 1 month after carotid occlusion.
RESULTS: Eight patients were moderate risk and 18 low risk. Mean follow-up was 15.3 months. Mean CT follow-up was 10.2 months. No low-risk patient developed a postocclusion ischemic deficit by examination or infarct by CT. One patient in the moderate-risk group developed right hemiparesis and a left posterior middle cerebral artery infarction by CT 2 months after carotid occlusion.
CONCLUSION: In this series, BTO combined with quantitative CBF analysis was a safe and reliable technique for identification of patients at risk for ischemic infarction after carotid occlusion, despite a normal clinical BTO.
Therapeutic internal carotid artery (ICA) occlusion is a common treatment in the management of symptomatic intracavernous carotid artery aneurysms (cavernous sinus aneurysms), giant ICA aneurysms, and certain skull base neoplasms. In the past, these lesions were often treated by means of open cervical carotid artery sacrifice, with or without cerebral revascularization, or by open intracranial approaches (1–4). Most centers now manage these lesions with endovascular carotid occlusion with or without cerebral revascularization.
Concurrent with the advancements in endovascular techniques, many diagnostic tests have been developed to evaluate the risk of ischemic infarction from carotid occlusion before permanent ICA sacrifice. The most widely accepted tool is the clinical balloon test occlusion (BTO) introduced by Serbinenko in 1974 (5). Before clinical BTO, approximately 25% of patients developed infarctions after carotid occlusion and 12% died as a result of abrupt carotid occlusion (6). By incorporating clinical BTO, the stroke rate after carotid occlusion has markedly improved. Yet despite passing a clinical BTO, a significant percentage of patients will still develop infarction as a complication of permanent carotid occlusion (6).
Other techniques have been combined with clinical BTO, including transcranial Doppler sonography, electroencephalography, quantitative cerebral blood flow (CBF) analyses (such as with xenon-133, xenon-enhanced CT, and positron emission tomography [PET H2O]), and qualitative CBF analyses (such as single photon emission CT [SPECT] with hexamethyl-propyleneamine oxime [HMPAO] and perfusion CT), in an attempt to identify those patients with compromised hemodynamics despite a normal examination (6–15). More recently, the addition of physiologic stressors to the current armamentarium (induced hypotension, acetazolamide, or CO2 challenges) have been suggested to aide in the identification of patients with compromised cerebrovascular reserve. However, confounding factors such as tumor-related neurologic deficit or infarction, subarachnoid hemorrhage or vasospasm related-infarction, and embolic infarction have made it difficult to determine whether such technologies aide in the prediction of ischemic infarction after carotid occlusion. As a result, these technologies have not been universally accepted, leaving some centers to advocate prophylactic extracranial-intracranial revascularization in all patients undergoing therapeutic carotid occlusion to minimize the risk of postocclusion ischemic stroke (16–17).
The aim of this study was to determine the outcome of carotid occlusion with selective cerebral revascularization in patients who had normal clinical BTO, by adding quantitative CBF by means of xenon-enhanced CT analysis during BTO (quantitative BTO) and focused endovascular BTO techniques.
Methods
A retrospective review of electronic and clinic records from our medical center identified 625 patients who underwent evaluation for therapeutic carotid occlusion between 1987 and 2001. Patient exclusion criteria were asymptomatic cavernous sinus aneurysms, carotid-cavernous fistula, tumor, previous stroke or history of cerebrovascular disease, extracavernous aneurysm or subarachnoid hemorrhage, craniotomy for open direct carotid occlusion, failed clinical BTO (high-risk patients), and BTO without preocclusion clinical BTO or quantitative BTO. Aneurysms were defined as cavernous only if they were found to arise proximal to the ophthalmic artery angiographically. Paraclinoidal aneurysms, arising at or above the ophthalmic artery, that extended into the cavernous sinus were excluded. A total of 27 patients with symptomatic cavernous sinus aneurysms underwent both a 15-minute clinical BTO and quantitative BTO. Twenty-six patients then underwent therapeutic carotid occlusion with or without extracranial-intracranial bypass. One patient did not undergo carotid occlusion secondary to markedly diminished CBF per xenon-enhanced CT (<30 mL/100 g/min) criteria and multiple severe systemic comorbidities making general anesthesia contraindicated. She was, therefore, also excluded from the study outcome analysis.
Patient Population
Of the 26 patients included in our study, six were male and 20 were female. Mean age was 60 years (range, 30–80 years). All 26 patients had symptomatic unilateral cavernous sinus aneurysms. Patient pretreatment signs and symptoms are presented in Table 1. All patients underwent and passed a 15-minute clinical BTO. Each patient then underwent a xenon-enhanced CT CBF analysis during balloon inflation followed by a baseline xenon-enhanced CT CBF analysis performed with the balloon deflated.
Comparison of signs and symptoms before and after carotid occlusion in 26 patients with symptomatic cavernous sinus aneurysms
Clinical and Quantitative BTO Techniques
The details of the 15-minute clinical BTO and quantitative BTO performed in this study have been described previously (18). In general, a four-vessel cerebral angiogram was obtained after a complete baseline neurologic examination. A double-lumen 5F Swan-Ganz catheter (Edwards Laboratories, Annasco, Puerto Rico) or double-lumen occlusion balloon catheter (Meditech, Watertown, MA) was then introduced into the femoral artery and advanced to the ICA at the level of the C1–2 vertebral bodies. A bolus of 7000 U of intravenous heparin was administered when the balloon catheter was introduced into the femoral artery. While measuring continuous distal ICA and systemic blood pressures, the endovascular balloon was inflated under direct fluoroscopic visualization. The balloon was expanded until the distal ICA pressure fell and/or the balloon deformed from a spherical configuration to a tubular vessel-like shape. Once arterial occlusion was complete, contrast material (Optiray; Mallinckrodt, Inc., St. Louis, MO) was injected through the distal catheter lumen under fluoroscopy to confirm cessation of anterograde flow. In the cases in which the vasculature was more tortuous (n = 2), the occlusion balloon was a “microballoon” type (NDSB; Target Therapeutics, Freemont, CA) and was positioned in the petrous portion of the ICA. However, when this balloon was used, no distal pressures could be measured. The patient then underwent a continuous neurologic evaluation for the first 5 minutes of balloon occlusion, followed by repeated examinations at 4–5-minute intervals. The neurologic evaluations were performed by clinical neurospecialists who maintained continuous verbal dialogues with the patients and were continuously evaluating patient strength, sensation, cognition, and cranial nerve function during the testing period. The balloon was kept inflated for 15 minutes but could be immediately deflated if neurologic deficits developed. If no neurologic deficits occurred during the 15 minute clinical BTO, the balloon was deflated but maintained in position and the patient was taken to the CT suite. The balloon was then reinflated, and a xenon-enhanced CT CBF study was obtained (inflated or quantitative BTO). The balloon was then deflated and removed. After 15 minutes, a repeat xenon-enhanced CT CBF study (baseline study) was performed. Methods of xenon-enhanced CT CBF measurements have been described previously (19).
Clinical and Quantitative BTO-Based Criteria for Patient Risk and Management
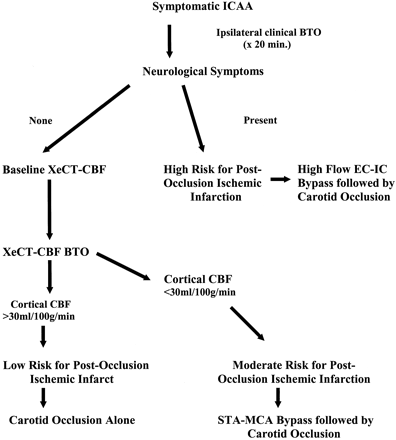
By using previously published criteria, patients were grouped into one of three categories based on a minimum CBF value in any vascular territory (6, 18). Patients were placed into the high-risk group if they failed clinical BTO. Patients were placed into the moderate-risk group if they passed the clinical BTO but developed quantitative BTO values less than 30 mL/100 g/min. Patients were placed into the low-risk group if they passed both the clinical and quantitative BTO. High-risk patients underwent high-flow revascularizations with saphenous vein or radial artery grafts followed by carotid occlusion. Moderate-risk patients underwent extracranial-intracranial revascularization, usually superficial temporal artery-to-middle cerebral artery bypass, followed by carotid occlusion. Low-risk patients underwent carotid occlusion alone. Our entire carotid occlusion with selective revascularization management protocol is depicted in Fig 1. The carotid occlusion technique itself was accomplished with endovascular balloons (Target Therapeutics) and coils (Cook, Bloomfield, IN). The balloons were placed exactly where the balloons used for the clinical and quantitative BTO were positioned, angiographically with the coils more proximal.
Management algorithm for symptomatic cavernous sinus aneurysms. ICCA indicates intracavernous carotid artery aneurysm; EC-IC, extracranial-intracranial; STA-MCA, superficial temporal artery-to-middle cerebral artery
Follow-up
Detailed neurologic examinations were obtained 1–4 weeks after carotid occlusion and then at 3–6-month intervals in all patients (mean, 15.3 months; range 3.4–39.4 months). All patients underwent routine neuro-ophthalmologic evaluations before and after carotid occlusion. Neurologic deficits were documented and compared with preocclusion deficits. Cranial neuropathies and other neurologic symptoms attributable to changes within the cavernous sinus were evaluated and compared with preocclusion findings during these same time intervals. All patients underwent CT neuroimaging at least 1 month after carotid occlusion (mean, 10.2 months; range, 1.1–39 months). Any postocclusion infarction documented by neuroimaging was considered a study end point and evidence of postocclusion infarction whether ischemic or embolic.
Results
Of 26 patients with symptomatic cavernous sinus aneurysms who passed clinical BTO, eight (31%) were considered moderate risk for postocclusion ischemic infarction. All eight patients then underwent superficial temporal artery-to-middle cerebral artery bypasses followed immediately by carotid occlusion. The remaining 18 patients were considered low risk for postocclusion ischemic infarction and underwent carotid occlusion alone. No immediate cortical or subcortical infarctions were identified in either the low-risk or moderate-risk group at CT, angiography, or clinical examination. Fifteen patients underwent cervical ICA BTO testing and 11 underwent petrous ICA BTO testing. No endovascular or perioperative complications occurred. All bypass grafts were confirmed patent by angiography at the time of carotid occlusion and by Doppler sonography at the time of discharge.
Delayed Infarctions and Cortical or Subcortical Deficits
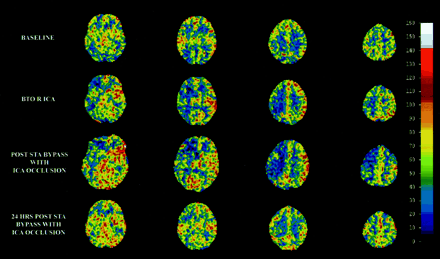
Follow-up neuroimaging identified a delayed infarction in one of the eight moderate-risk patients and in none of 16 low-risk patients. None of the low-risk patients developed delayed symptomatology suggestive of postocclusion ischemia or infarction. In the moderate-risk group, only the patient with delayed radiographic infarction developed symptoms. This patient was an 80-year-old woman with a symptomatic left cavernous sinus aneurysm. She had an unremarkable clinical BTO, but baseline and quantitative BTO showed decreased perfusion bilaterally with the left ICA distribution being most affected (Fig 2). The patient underwent a left superficial temporal artery-to-middle cerebral artery bypass followed by an endovascular petrous carotid occlusion. The patient had an unremarkable postoperative course, but developed right-sided weakness approximately 6 months after carotid occlusion with bypass. CT confirmed a new left posterior middle cerebral artery infarction corresponding to the patient’s symptoms (Fig 3).
Example of a xenon-enhanced CT CBF analysis for a patient at moderate risk for ischemic infarction after carotid occlusion. Quantitative BTO CBF analysis (BTO R ICA) identified decreased perfusion of the right ICA. The patient then underwent cerebral revascularization followed by carotid occlusion, with restored baseline CBF postoperatively. STA indicates superficial temporal artery. Each column represents a different brain level of CBF 5 min apart.
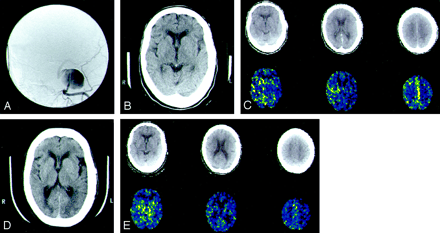
Postocclusion infarction predicted by quantitative BTO in an 80-year-old woman who presented with left third, fourth, and sixth nerve palsies.
A, Digitally subtracted cerebral angiogram of the ICA (AP view) reveals a left cavernous ICA aneurysm. The patient underwent clinical BTO and did not develop neurologic symptoms.
B, Baseline and C, subsequent quantitative BTO by means of xenon-enhanced CT CBF analysis reveal decreased perfusion in the left ICA distribution, as well as worsening hemispheric asymmetry with temporary BTO. The patient then underwent a left superficial temporal artery-to-middle cerebral artery bypass followed by carotid occlusion.
D and E, Repeat images obtained at 6-month follow-up show a new left posterior middle cerebral artery branch infarction.
For B and C, top row shows correlative CT image level of CBF measurement and bottom row represents xenon-enhanced CT-CBF analysis.
Signs and Symptoms Related to Cavernous Sinus Aneurysm
Of the 26 patients with cavernous sinus aneurysms, 24 (92%) initially presented with diplopia secondary to cranial neuropathies, six (23%) with severe headache, five (19%) with a Horner’s syndrome, three (12%) with trigeminal symptomatology, and three (12%) with “blurred vision” (Table 1). The most common cavernous cranial neuropathy involved the abducens nerve (69%), whereas the trigeminal nerve was least affected. After carotid occlusion, seven patients (27%) described the development of new symptoms, worsening of existing symptoms, or were found to have new cranial neuropathies on examination (Table 2). During this same period, however, 25% of patients noted improvement in their diplopia, 66% were found to have improved visual ability to focus, and 33% noted improvement in headache (Table 1).
New signs and symptoms after endovascular carotid occlusion in patients with cavernous sinus aneurysms
At latest follow-up (mean, 15.3 months; range, 1.1–39 months), most of the new signs and symptoms identified in the initial postocclusion period had resolved. Two-thirds of patients had noted improvement in their diplopias, with nearly 90% of all abducen palsies improved (Table 1). All patients presenting with “blurred vision” described improved visual clarity, and 50% of patients presenting with headache noted improvement. However, patients presenting with complete ophthalmoplegia or trigeminal sensory symptoms were less likely to obtain clinically significant improvement in their symptoms. Only two patients had postocclusion signs and symptoms not identified before carotid occlusion. One patient developed complete ophthalmoplegia after presenting with a partial occulomotor paresis, and one patient developed a hypoglossal paresis after carotid occlusion that was not identified on preocclusion testing (Table 2). The ophthalmoplegia, which developed in the early postocclusion period, likely represented ischemic or compressive injuries to the structures within the cavernous sinus due to aneurysm swelling and thrombosis after occlusion. As new microcollateral vessels to the cavernous sinus structures form and the acute thrombotic inflammatory response resolves, these neurologic deficits might improve. The hypoglossal paresis cannot be described by a local mass-effect phenomenom within the cavernous sinus, but rather may reflect an embolic injury to the microvasculature supplying the hypoglossal nerve at the time of carotid occlusion.
Fifteen patients had postocclusion follow-up of more than 1 year’s time. Fourteen (93%) of these 15 patients had improvements in their preocclusion symptoms. One patient, who presented with “blurred vision” and a partial occulomotor paresis, developed complete ophthalmoplegia after carotid occlusion. Her subjective vision improved, but her ophthalmoplegia did not. Five patients had persistent partial occulomotor nerve palsies (all were improved from before carotid occlusion), and three had abducen pareses (all improved from before carotid occlusion). Eight patients were completely asymptomatic, with normal neurologic examinations.
Discussion
Since Matas’ (20) initial description of manual common carotid artery compression in the preoperative evaluation of carotid occlusion risk, several groups have devised tests to either directly or indirectly evaluate cerebrovascular reserve for those patients who require carotid sacrifice (7, 18, 21–26). Although a clinical BTO is accepted by most centers as a means to screen for patients at highest risk of postocclusion ischemic infarction, the value of additional tests in their ability to identify those at moderate risk remains a topic for debate. Some studies identify postocclusion ischemic complications as high as 44% despite clinical BTO and CBF and/or cerebral perfusion BTO assessments, while others quote rates of less than 5% by using similar technologies (12, 13, 27–29). Likewise, the benefit of cerebral revascularization in patients requiring carotid occlusion is controversial. In Sen and Sekhar’s (30) series of 30 patients with skull base lesions requiring carotid occlusion, all 30 patients also underwent revascularization procedures, with 12 developing ischemic infarctions. However, in the Mayo clinic series (31) the rate of ischemic infarctions was far less (12% during a 13-year period, n = 25 of 202).
The reasons for such variability in the outcome of patients requiring carotid occlusion are likely due to three factors: patient selection, management paradigms, and methods of outcome measures. In our study, every attempt was made to minimize confounding variables so as to truly assess our ability to screen patients. As a result, all patients with carotid-cavernous fistulas, tumor, previous stroke or history of cerebrovascular disease, extracavernous aneurysm or subarachnoid hemorrhage, or craniotomy at time of carotid occlusion were excluded from our study. All patients in this study underwent the management paradigm outlined in Fig 1.
Without exception, outcomes were measured at several time points by both clinical and radiographic criteria to account for acute, subacute, and delayed events related to carotid occlusion with selective revascularization. An example of moderate-risk patient is displayed in Fig 2. Because of our strict inclusion criteria, only a small percentage of patients evaluated for carotid occlusion at our center was included in this study, as most lesions were not cavernous sinus aneurysms. Furthermore, most of the cavernous sinus aneurysms did not require neurosurgical intervention, as defined by Linskey et al (32), and thus were not included in the study. As opposed to previously published series on this topic, our selection process minimized the risk of confounding variables within our patient population and resulted in the largest series focusing on the specific problem of identifying patients at hemodynamic risk for stroke after carotid occlusion.
Local Cavernous Sinus Aneurysm Symptom Control after Carotid Occlusion
Treatment indications in our study for patients with cavernous sinus aneurysms were based on the Linskey et al (32) retrospective study of the natural history of cavernous sinus aneurysms. Indications for treatment included symptomatic idiopathic cavernous sinus aneurysms that were defined by progressive ophthalmoplegia or visual loss, severe ipsilateral facial or orbital pain, or demonstrable aneurysm enlargement based on imaging criteria.
Unlike previously published series on the management of cavernous sinus aneurysms, we evaluated patient symptoms before treatment, early after treatment, 1 year after treatment, and at last evaluation. Twenty-seven percent of the patients in our series developed new symptoms or had progression of their preocclusion symptoms in the early postocclusion period. These symptoms often lasted several weeks and generally resolved within 1–3 months. At last follow-up, only two patients had persistent neurologic deficits not identified by preocclusion testing. These temporary symptoms developing in the early postocclusion period likely represent ischemic or compressive injuries to the structures within the cavernous sinus owing to aneurysm swelling and thrombosis after occlusion. With resolution of the acute thrombotic process, neurologic deficits usually improved. In addition, 93% of patients at 1 year after carotid occlusion had either complete resolution or marked improvement of preocclusion symptoms.
Role of Quantitative CBF Analyses in Evaluating Hemodynamic Risk
The goal of an ancillary study to a clinical BTO is to allow for selective bypass surgery by identifying the patient that develops a near exhaustion of hemodynamic reserve with carotid occlusion from the patient who does not. Presumably such an individual could experience infarction with any additional compromise of perfusion pressure or collateral supply, both of which commonly occur during sleep with a reduction of pressure and a potential rise of Pco2. The paradigm chosen for this report identified patients with a quantitative CBF in whom BTO reduced flow from a norm of 50 down to a range of 20–30 mL/100g/min. The physiologic reliability of this strategy was supported in the report by Witt et al (19) of CBF changes that accompanied BTO in 156 patients who passed clinical BTO. In that report, no patient had a middle cerebral artery flow value below 20 mL/100g/min, although 14 patients developed flow in the 20–30 mL/100g/min range. This strategy has been further validated by the recent work of Marshall et al (33) in which they prospectively evaluated the predictive validity of quantitative CBF measurements during ICA BTO by using intracarotid xenon-133 injection and scalp scintillation recordings. In their study, a CBF of less than 30 mL/100 g/min during BTO was the only variable predictive of stroke after carotid occlusion. They found that the negative and positive predictive values for quantitative CBF findings were far better than those for clinical neurologic examination or sustained-attention tests.
Although many strategies have been used to identify patients at hemodynamic risk, few clinical series have been reported in which such information was used in guiding treatment options. The report by Origitano et al (27) with a greater than 40% associated stroke rate provides an example of the many potential confounders that can complicate the predictive power of BTO testing. Their use of a qualitative CBF method was also problematic because of the inability of such a study to distinguish with confidence the group that drops flow unilaterally (19). Quantitative CBF analyses, however, remove the potential problems seen with qualitative CBF evaluations when low-flow states exist bilaterally, as seen with our patient in Fig 3. With use of qualitative measures, this patient, who later developed ischemic infarction, would not have been identified as having hemodynamically compromised CBF, as hemispheric flow was fairly symmetric bilaterally. Thus, the use of qualitative measures such as perfusion CT, diffusion or perfusion MR imaging, or SPECT HMPAO may not adequately predict ischemic risk in patients with bihemispheric or multiterritorial disease.
Although xenon-enhanced CT has been used extensively in the past, it is currently only available in the United States as an IND experimental status technology, as is PET CBF analysis. It is anticipated that both of these technologies, as well as Xe133 CBF and Xe133 SPECT (which are approved by the U.S. Food and Drug Administration), will become models for quantitative measures of CBF in the future, to which our results can be applied. However, since xenon-enhanced CT has only recently been approved for experimental use in humans in the United States, our results can be and should be applied when using alternative technologies that measure quantitative CBF such as PET quantitative CBF, intraarterial Xe133 CBF, and Xe133 SPECT (34). Technologies such as transcranial Doppler sonography have also been used in the guided management of carotid occlusion, but because transcranial Doppler sonography measures blood velocity it can only indirectly estimate tissue CBF (35). Therefore, our results may not be applicable to its use.
Ischemic Infarction After Carotid Occlusion with Selective Revascularization
In our series, no patient identified as being low risk by clinical BTO plus quantitative BTO developed symptomatic or radiographic evidence of ischemic infarction with a mean clinical follow-up of more than 15 months and radiographic follow-up of more than 10 months. Thus, the combination of clinical plus quantitative BTO in the evaluation of these patients resulted in a 0% false-negative rate. When comparing our results with those of other previously published series, this combination of clinical plus quantitative BTO also appears to be more sensitive in identifying patients who do not require revascularization when performing carotid occlusion than is clinical BTO alone (6, 12, 13, 27–29). Unfortunately, a true- or false-positive rate could not be determined with this protocol as all moderate-risk patients underwent extracranial-intracranial bypass surgery and thus were presumably no longer as severely hemodynamically challenged. Because no previously published series excluded these potential confounders, they could not assess the true risk of infarction secondary to carotid occlusion in patient groups by using BTO and/or other physiologic technologies.
Embolic Infarction After Carotid Occlusion
Thromboembolic infarction is a common cause for neurologic deficit after carotid occlusion and is reported to occur in 5–10% of patients, despite a normal BTO (15). Furthermore, risk for thromboembolic infarction cannot be assessed by using quantitative BTO or other hemodynamic studies. The use of periprocedural anticoagulation and postocclusion antiplatelet therapies have reportedly decreased this risk over the past 10 years, as has the increased utilization of the endovascular technique to perform carotid occlusion (2, 36–38). Yet, despite the incorporation of these modalities, embolic infarction is still reported.
In our series, all patients underwent periprocedural anticoagulation by using a 7000-U intravenous heparin bolus administered during balloon catheter insertion for BTO or carotid occlusion. Daily aspirin (325 mg orally) was then started 24 hours after carotid occlusion with or without revascularization and continued for life. In ddition, careful attention was made to duplicate the technique of carotid occlusion between the BTO and permanent occlusion procedures. Although, in our patients a combination of endovascular detachable balloons, nondetachable balloons, and coils were used in the carotid occlusion technique, permanent occlusions were performed as proximal to the aneurysm neck as possible. Previous literature has suggested that by decreasing the dead space you may decrease the risk of thomboembolism presumably by reducing the likelihood of collateral recanalization of the aneurysm (39). In the few patients in whom the BTO was performed in the cervical ICA, the distal vessel and aneurysm were injected with contrast material to confirm flow stasis. If contrast material washout was seen, the balloon was advanced further and the process repeated until contrast material stasis was confirmed. Then, the clinical and quantitative BTOs were performed. When the patients later underwent permanent carotid occlusion, the balloon(s) and/or coils were placed at the location where the BTO was performed. We believe this was important in minimizing the risk of both ischemic and embolic infarction by ensuring aneurysm flow stasis. If endovascular carotid occlusion were performed more distally than where BTO was studied this could have resulted in occlusion of collateral vessels not occluded during BTO, causing unpredicted infarction. Similarly, more proximal occlusion could have resulted in collateral flow via vessels that were occluded during BTO, resulting in persistent aneurysm filling. As a result of our method of carotid occlusion and BTO testing, no resultant embolic infarctions were seen and all aneurysms were thrombosed at last angiographic evaluation. Thus, we advocate performing test occlusion analyses at the location of the expected permanent occlusion.
Conclusion
In this series, BTO combined with quantitative CBF was a safe and reliable technique for identification of patients with cavernous sinus aneurysms at risk for ischemic infarction after carotid occlusion despite normal clinical BTO. Quantitative CBF is an important measurement capable of distinguishing patients at various stages of ischemic risk. Although quantitative stable xenon-enhanced CT is not available at this time in the United States, the strategies employed in this series can be extrapolated to other quantitative technologies. Further, approximately one in four patients undergoing carotid occlusion with selective revascularization will have worsening of their symptoms or develop new symptoms within the first month of permanent carotid occlusion. However, over 90% will have marked improvement in their symptoms by 1 year after carotid occlusion.
Footnotes
Supported in part by an educational grant through Diversified Diagnostic Products, Inc.
References
- Received June 19, 2002.
- Accepted after revision January 21, 2003.
- Accepted after revision January 21, 2003.
- Copyright © American Society of Neuroradiology