Abstract
BACKGROUND AND PURPOSE: Fluid-attenuated inversion recovery (FLAIR) MR imaging sequences have been previously described in the evaluation of acute subarachnoid hemorrhage (SAH) in human subjects and have demonstrated good sensitivity. The purpose of this study was to evaluate a FLAIR sequence in an animal model of SAH and to compare the results with those obtained with non–contrast-enhanced CT.
METHODS: SAH was experimentally induced in 18 New Zealand rabbits by injecting autologous arterial blood into the subarachnoid space of the foramen magnum. Nine animals had high-volume (1–2 mL) injections, and nine animals had low-volume (0.2–0.5 mL) injections. Four control animals were injected with 0.5 mL of saline. The animals were imaged with a FLAIR sequence and standard CT 2–5 hours after injection. Gross pathologic evaluation of seven of the animals was performed. Four blinded readers independently evaluated the CT and FLAIR images for SAH and graded the probability of SAH on a scale of 1 to 5 (1 = no hemorrhage, 5 = definite hemorrhage).
RESULTS: Overall, the sensitivity of FLAIR was 89%, and the sensitivity of CT was 39% (P < .01). In animals with a high volume of SAH, the sensitivity of FLAIR was 100%, and the sensitivity of CT was 56%. In animals with a low volume of SAH, the sensitivity of FLAIR was 78%, and the sensitivity of CT was 22%. The specificity of FLAIR in animals without SAH was 100%, and the specificity of CT was 100%. The average reader score for FLAIR was 3.8, and that for CT was 2.2 (P < .001). Reader scores for FLAIR were higher than those for CT in 94% (P < .01) of animals with SAH and in 25% of animals without SAH (P > .05). Seven animals underwent gross pathologic examination, and all had blood in the subarachnoid space around the brain stem.
CONCLUSION: FLAIR was more sensitive than CT in the evaluation of acute SAH in this model, especially when a high volume of SAH was present. This study provides a model for further experimentation with MR imaging in the evaluation of SAH. These findings are consistent with those of current clinical literature, which show FLAIR to be an accurate MR sequence in the diagnosis of SAH.
Fluid-attenuated inversion recovery (FLAIR) imaging has been reported to be a sensitive and specific method in the diagnosis of disease of the subarachnoid space. In FLAIR MR imaging, an inversion recovery pulse sequence is used with an inversion time that suppresses the signal of CSF but provides relative T2-weighted images. In the acute phase, blood products in the CSF cause both T1 and T2 shortening. In the CSF, these competitive effects null the signal from subarachnoid hemorrhage (SAH). Additionally, CSF pulsation results in artifactual signal. As a result, conventional MR images do not reliably depict SAH; however, FLAIR sequences suppress CSF signal and allow SAH to be depicted (1–6). Specifically, recent reports suggest that FLAIR imaging may be as sensitive or more sensitive than CT in the diagnosis of SAH; however, most studies have involved small numbers of patients. Furthermore, to our knowledge, the sensitivity of FLAIR relative to that of CT has not been studied in an animal model. The purpose of our study was to compare FLAIR MR imaging and CT in a rabbit model of SAH.
Methods
An SAH model was created by injecting autologous arterial blood into the subarachnoid space at the foramen magnum of 22 New Zealand rabbits with a technique previously described (7, 8). Direct puncture was performed percutaneously with a 23-gauge needle by using anatomic landmarks. The position in the subarachnoid space was confirmed by the observation of free flow of CSF into the needle hub. Animals were injected with 0.2–2.0 mL of blood drawn from the dorsal artery of the ear immediately prior to injection. The animals were divided into five groups, with each group receiving a different volume of injected blood. Four animals received 0.2 mL (group 1), five animals received 0.5 mL (group 2), five animals received 1.0 mL (group 3), and four animals received 2.0 mL (group 4). Additionally, 0.5 mL of normal saline was injected into four animals without SAH (group 5). The animals were euthanized to minimize artifacts from gross motion and to determine if use of this sequence were feasible even with ideal conditions. Each remained in Trendelenburg position for 30 min after the injection to allow blood to enter the cranial CSF.
All animals undewent MR imaging (1.5-T) 3–5 h after injection, with a FLAIR sequence performed in the axial and sagittal planes (10,000/150/2500 [TR/TE/TI]; section thickness, 4 mm; field of view, 220 × 220; matrix, 210 × 250; standard head coil). Brain CT (section thickness, 3 mm; field of view, 100; window width, 200 HU; window length, 40 HU) was performed immediately after MR imaging. Seven animals underwent autopsy with direct gross pathologic evaluation of the location of the hemorrhage.
Four neuroradiologists (D.F.K., H.M.D., M.E.J., J.S.) interpreted the CT and MR studies. All readers were blinded to the presence of SAH. CT and MR images were evaluated independently. Readers graded the probability of the presence of SAH on a scale of 1 to 5, with 1 indicating no hemorrhage and 5 indicating definite hemorrhage. Data were compiled, and MR and CT findings were compared by using the paired two-tailed t test, χ2 test, and κ statistic.
Results
Overall, including all animals with SAH, the sensitivity of FLAIR was 89% (16/18), with a true-positive finding being an average reader score of 3 or greater. With the same criterion, the sensitivity of CT was 39% (7/18) (P < .01). In animals with a high volume of SAH (groups 3 and 4), the sensitivity of FLAIR was 100% (9/9), and the sensitivity of CT was 56% (5/9). In animals with a low volume of SAH (groups 1 and 2), the sensitivity of FLAIR was 78% (7/9), and the sensitivity of CT was 22% (2/9). The specificity of FLAIR in animals without SAH (group 5) was 100% (4/4), and the specificity of CT was 100% (4/4). The Table summarizes these results.
The positive predictive value of finding SAH by FLAIR was 100%. The positive predictive value for CT was also 100% (ie, no positive results in the non-SAH group). The negative predictive value of FLAIR was 67%, and that of CT was 27%. Overall, the accuracy of FLAIR was 91%, and the accuracy of CT was 50%.
Overall, in animals with SAH (groups 1–4), the average reader score for FLAIR was 3.8, and that for CT was 2.2 (P < .001). In animals without SAH (group 5), the overall average score was 1.8 for FLAIR and 2.0 for CT (P > .05) (Fig 1). Reader scores for FLAIR were higher than those for CT in 94% (17/18, P < .01) of animals with SAH and in 25% (1/4) of animals without SAH (P > .05). In animals that received an injection of a large volume of blood (groups 3 and 4), the average reader score with FLAIR was 4.8, and that with CT was 2.7 (P < .001) (Fig 2). In animals with a low volume of blood (groups 1 and 2), the average score with FLAIR was 2.8, and that with CT was 1.6 (P < .001) (Fig 3).
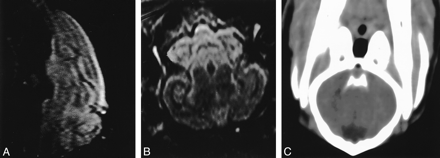
Brain images in a control animal.
A and B, Sagittal (A) and axial (B) FLAIR MR images (10,000/150/2500 [TR/TE/TI]) show no evidence of increased signal in the subarachnoid space.
C, CT image demonstrates no hyperattenuation to suggest SAH.
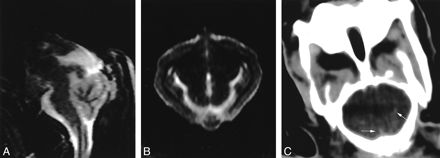
High-volume injection of blood to induce SAH.
A and B, Sagittal (A) and axial (B) FLAIR MR images (10,000/150/2500) show hyperintense signal throughout the subarachnoid space; this finding is consistent with hemorrhage.
C, CT image shows linear areas of increased attenuation (arrows) probably due to SAH
Low-volume injection of blood to induce SAH.
A and B, Sagittal (A) and axial (B) FLAIR (10,000/150/2500) MR images show hyperintense signal throughout the subarachnoid space, consistent with hemorrhage.
C, CT image shows a linear area of increased attenuation (arrowhead), which may be due to SAH or streak artifact.
In the seven animals that underwent gross pathologic examination, all had a clot in the subarachnoid space around the brain stem.
Analysis of variability between readers with the κ statistic showed a statistically significant reproducibility for both CT (P < .01, κ = 0.37) and FLAIR (P < .001, κ = 0.43) imaging.
Discussion
The diagnosis of SAH has historically relied on non–contrast-enhanced CT or lumbar puncture. While these techniques have been effective for this purpose, comprehensive examination of patients with an acute onset of neurologic deficits or severe headache requires a more comprehensive diagnostic evaluation, typically one involving MR imaging and MR angiography. Ideally, MR imaging could be used for this comprehensive examination and could reliably depict acute SAH; however, at present, MR imaging has not been proven effective for this purpose, either in human or animal studies.
The diagnosis of acute SAH with conventional spin- or gradient-echo MR sequences has been difficult because of the small effect of SAH on CSF signal intensity (unlike the effect of blood products in the parenchyma) and MR artifacts. Findings of in vitro studies have suggested that conventional MR imaging is not acceptable for the depiction of SAH because of hemoconcentration, clot retraction, CSF pulsation, and the relatively high oxygen saturation of hemoglobin in the subarachnoid space as compared with that of the brain parenchyma (1, 9). Findings of clinical studies have suggested that conventional MR sequences (spin- and gradient-echo sequences) are reliable and sensitive for SAH (6, 10–12). These findings remain the subject of debate, and most centers rely on CT for the imaging diagnosis of SAH (6). However, CT does have limitations. In particular, CT has decreased sensitivity in areas of increased bone density secondary to artifact (beam hardening and streak artifacts). Such artifacts do not limit evaluation of the posterior fossa with MR imaging. Also, small amounts of blood diluted by CSF might not be attenuated on CT scans. Yet, CSF does not decrease MR signal intensity, and therefore, it shows SAH because of differences in T1 and T2.
Acute hemorrhage in the subarachnoid space results in shortening of both T1 and T2 (1, 13). Some might expect that this effect would allow differentiation of adjacent gray matter and surrounding CSF. However, the effects are competitive on conventional spin-echo images. On short-TE images, CSF shows increased signal intensity due to T1 shortening, as compared with that of gray matter. However, T2 effects due to the presence of deoxyhemoglobin, though they are less than the effects with parenchymal hemorrhage, are present and result in decreased signal intensity. These effects make differentiation of CSF from gray matter difficult. Long-TE images have additional problems. High-signal-intensity CSF on images obtained with these sequences is difficult to separate from hemorrhage, which has slightly increased signal intensity compared with that of the gray matter. Also, pulsation artifacts from the CSF are prominent on long-TE images and further impair detection (14). Gradient-echo images are sensitive to paramagnetic states of hemoglobin oxygenation; however, gradient-echo studies may not be effective in the acute setting because little deoxyhemoglobin is acutely present in SAH (2, 15).
Study findings have shown that if CSF signal intensity is suppressed on T2-weighted images (FLAIR images), acute hemorrhage in the CSF is detectable (3–5, 9, 16). An inversion time (generally a long TI on the order of 1800–2200 ms) can be selected to null CSF signal (also reducing pulsation artifact) (17). A relatively long TE allows for T2 weighting. Because the T2 of gray matter is shorter than the T2 of subarachnoid blood, the two can be distinguished. Additionally, T1 shortening in SAH causes relatively increased signal intensity in the subarachnoid space. With this technique, SAH has increased signal intensity compared with that of the brain, and the CSF is no longer seen.
Clinical studies have been conducted to compare FLAIR and CT in SAH detection. Atlas (9) reported findings from a series in which FLAIR imaging was superior to CT in SAH detection and produced greater conspicuity of the hemorrhage in most cases. Noguchi et al (3) reported findings of three cases in which FLAIR images demonstrated SAH within 5 h of its onset. The same group later reported findings in 20 patients in whom SAH was identified by using FLAIR imaging, with an accuracy of 100% in the acute setting (5). These data agree with our findings that FLAIR imaging is exquisitely sensitive to SAH. However, we believe that caution must still be exercised in using FLAIR imaging for this indication, because an inexperienced reader may misdiagnose pulsation artifact from CSF, particularly in the posterior fossa.
These clinical studies have relied on CT as a criterion standard for diagnosis. While this is accepted in the clinical setting, an in vivo model allows direct pathologic evaluation to be the criterion standard. By their nature, clinical studies are unable to control for variables such as hemorrhage volume or location. Using this model, we were able to investigate whether FLAIR was as good as or better than CT for SAH detection. Also, we were able to show that a model of SAH that is simple, reproducible, and accepted in the neurosurgical literature may be used for imaging studies of SAH.
Another limitation of clinical studies is that most to date have been performed at 0.5 T. Increased field strength results in increased signal intensity and increased T2 susceptibility, both of which might dramatically change accuracy. For example, the small amount of deoxyhemoglobin present may have a greater effect on signal intensity with an increased field strength, potentially reducing sensitivity. However, our findings show that, at field strengths of 1.5 T, the superiority of FLAIR imaging is maintained.
Several investigators recently evaluated the use of susceptibility-weighted images for the detection of acute SAH, with some reported success in small series. In one recent series (18), T2*-weighted images were reported to be more sensitive in an acute setting, which was defined as imaging at less than 4 days after presentation. Such sensitivity might be expected several days after hemorrhage, because the amount of deoxyhemoglobin increases. However, depiction in the clinically relevant hyperacute period (0–24 hours) would be limited. In a second study (19), evaluation of FLAIR and T2* images in stroke showed that T2* images are superior in acute intraparenchymal hemorrhage, but FLAIR images are superior for the depiction of SAH. A third small in vitro study (20) in which FLAIR and susceptibility-weighted sequences were evaluated primarily for the depiction of intraparenchymal hemorrhage revealed that use of the susceptibility images to identify SAH was advantageous in several animals that had SAH in addition to parenchymal hemorrhage. To our knowledge, susceptibility-weighted images have not been evaluated systematically and might be severely limited in the posterior fossa, where they may be expected to have their worst susceptibility or blooming artifacts (13, 21, 22). This factor might limit their use in a manner similar to the use of CT images. The use of such sequences remains at best unproved, and while controversial, FLAIR imaging might prove essential for the diagnosis of SAH, as the literature suggests (23). Additionally, in areas of bone-tissue interface (ie, the brain surface), susceptibility artifact occurs, making the distinction of brain parenchyma and CSF difficult.
Several limitations were present in our study. An animal model is not a perfect replica of the human body. In the rabbit, the relatively small intracranial compartment, compared with surrounding skull, creates a large amount of bone artifact on CT images. This model was, therefore, used to evaluate CT in its least sensitive application, that is, in areas of high bone density. However, this is an excellent replica of the human posterior fossa. Previous reports have suggested that CT is not sensitive to hemorrhage in the posterior fossa secondary to streak artifact (3). This limitation represents a potential advantage of MR imaging.
Euthanized animals were used in this study to allow preliminary evaluation of the FLAIR sequence in these small animals in a controlled manner. We were able to minimize artifacts from gross movement and to avoid difficulties in maintaining the animals. The main limitation of this method is that CSF pulsation artifact is eliminated. Live animals may show a decrease in conspicuity of the SAH because of the pulsation; however, we expect that this contribution is small. Live animals may also show artifactual increased signal intensity from CSF pulsation, and this may be problematic. Future studies need to be performed in live animals to confirm our results.
We did not assess the change in signal intensity over time, which may be significant, considering blood product evolution. Studies of parenchymal hemorrhage indicate that 2 to 5 h pass until deoxyhemoglobin and methemoglobin form. This would suggest that blood product evolution would alter signal intensity during this time. In the subarachnoid space, these blood product concentrations are different, with more T2 shortening and reduced signal intensity. The implications of this for FLAIR imaging of CSF blood products in vivo is not well described.
The specificity of FLAIR imaging was not addressed in this study. Atlas (9) suggested that, to some extent, FLAIR imaging is not specific for SAH, since other causes of elevated CSF protein levels may increase signal intensity. To distinguish the possible causes, the clinical setting is important, as are the pattern of signal intensity abnormality and contrast enhancement. Furthermore, MR imaging might elucidate the cause of nonhemorrhagic subarachnoid space disease, allowing for a single noninvasive study for this indication.
Our control animals were those with cisternal puncture and no SAH injection, not animals without any puncture. In a worst-case scenario, traumatic puncture could have confounded our data by producing false-positive studies. This would have necessitated the use of animals without puncture. One control animal had a higher score with FLAIR imaging than with CT; however, even in this animal, the finding was not considered positive with either FLAIR imaging or CT. Because the resultant specificity of both techniques was 100%, examination of a group of animals without puncture was not necessary.
We did not evaluate susceptibility- or T2*-weighted images in this study. The investigators believed that this study represented an effort to evaluate FLAIR images specifically, as recent findings in the literature suggest that this might be the most sensitive single sequence for the evaluation of SAH and that the evaluation of additional sequences would not directly address this point. Additionally, our hypothesis was that MR imaging might offer an advantage compared with CT, particularly in the posterior fossa, for which the rabbit brain is a good model. It is in this area that susceptibility-weighted images are limited to the greatest degree by severe blooming artifacts. At present, while we recognize that some practitioners may use a susceptibility sequence in the acute setting, we do not use such images to depict acute hemorrhage, and our anecdotal experience suggests they are not effective for this purpose because of limitations in the posterior fossa.
Our data confirm what has been suggested in the literature, namely that FLAIR MR imaging is sensitive to hemorrhage in the subarachnoid space and that it is, in fact, more sensitive than CT in this model. In addition, this study involved a model that allows controlled evaluation of SAH with MR imaging. This model allows future evaluation of the FLAIR sequence and other imaging techniques.
Conclusion
In our study, FLAIR images were more sensitive than CT images in the detection of SAH. FLAIR imaging was superior to CT regardless of the volume of blood injected. In addition, FLAIR images accurately excluded hemorrhage in animals without SAH. The findings support the concept that FLAIR imaging is a viable technique for assessing SAH in patients.
Footnotes
1 Address reprint requests to Richard J. Woodcock, Jr, MD, Division of Neuroradiology, Department of Radiology, Emory University, 1364 Clifton Road NE, Atlanta, GA 30322.
References
- Received December 1, 2000.
- Accepted after revision May 8, 2001.
FLAIR MR imaging versus non–contrast-enhanced CT for the evaluation of SAH in rabbits: summary of accuracy
- Copyright © American Society of Neuroradiology