Abstract
BACKGROUND AND PURPOSE: Fibrinolysis with local intraarterial urokinase infusion for basilar artery thrombosis has been associated with a low rate of spontaneous symptomatic cerebral hemorrhage, even when patients have been treated late in the course of symptoms. Because urokinase is presently unavailable in the United States, this study was undertaken to determine the frequency of spontaneous cerebral hemorrhage in basilar artery fibrinolysis performed with tissue plasminogen activator (tPA).
METHODS: In a retrospective review of our initial experience with cerebral fibrinolysis for acute stroke using intraarterial tPA, four cases of basilar thrombosis were identified. Doses of the fibrinolytic agent and heparin, angiographic findings, clinical courses, and bleeding complications for these patients were determined. These results were compared with those from a prior study of 20 similar consecutive patients treated with urokinase.
RESULTS: Symptom duration before treatment was unlimited. Intraarterial doses of tPA were 20 to 50 mg. Patients received full systemic anticoagulation with heparin. Complete basilar artery recanalization was achieved in 75% of patients. Two patients treated with tPA had angioplasty and stent placement for related high-grade stenosis. Spontaneous symptomatic cerebral hemorrhage occurred in three (75%) of the four tPA-treated patients and in three (15%) of the 20 urokinase-treated patients.
CONCLUSION: The cerebral hemorrhage complication rate for intraarterial fibrinolysis with tPA was very high in cases of basilar artery thrombosis at the doses we used. Protocol adjustments should be considered.
Basilar artery thrombosis has been treated in our center and in many other centers with intraarterial urokinase (Abbokinase, Abbott Laboratories, N Chicago, IL) (1–8). Despite delays in diagnosis and treatment common in this condition, fibrinolysis with urokinase has had an acceptable safety profile, especially in view of the high mortality rate associated with conservative treatment. Spontaneous symptomatic cerebral hemorrhages occurred in 15% of our basilar thrombosis patients treated with intraarterial urokinase (6). Survival and neurologic outcomes for these patients appeared to improve relative to historical control subjects treated conservatively.
In 1999, production of urokinase was suspended by the United States Food and Drug Administration (FDA) (9, 10). At the time, there was limited experience using fibrinolytic agents other than urokinase for local intraarterial fibrinolysis in cases of basilar thrombosis or acute thromboembolic stroke (11–19). Previous experience with tissue plasminogen activator (tPA) (alteplase, Activase, Genentech, S San Francisco, CA) in patients with acute stroke was primarily with intravenous administration of the drug (20–22). Efficacy of administering tPA in the setting of acute stroke was demonstrated in one prospective study only when the drug was given intravenously within the first 3 hours of symptom onset (21). Hemorrhagic complications occurred more frequently when tPA was given after the first 3 hours (22).
Most patients with basilar artery thrombosis present and are referred for treatment after the 3-hour time window for delivery of intravenous tPA has expired. Furthermore, the data reveal better efficacy for recanalization of clots in proximal cerebral vessels using local intraarterial rather than systemic intravenous administration of fibrinolytic agents (13). Thrombolytic therapy for basilar thrombosis is, for these reasons, generally performed using local intraarterial delivery of the drug. On the basis of the available previously published data on local intraarterial tPA infusions for acute thromboembolic stroke, we substituted tPA for urokinase for the treatment of basilar artery thrombosis after urokinase supplies were exhausted. After noting a possible increase in hemorrhagic complications in our initial experience with tPA, we compared outcomes and complications to determine whether protocol adjustments were required.
Methods
Beginning in April 1999, tPA was substituted for urokinase for intraarterial fibrinolysis in cases of acute thromboembolic stroke. The protocol specified a maximum infusion of 25 mg tPA per hour and 50 mg per treatment. The drug was mixed in normal saline to achieve a concentration of 0.2 mg tPA/mL, the lowest concentration recommended by the manufacturer. Other aspects of the protocol previously in place specifying urokinase as the fibrinolytic agent remained unchanged, including concomitant intravenous delivery of heparin and no exclusion of angioplasty and stent placement for related high-grade stenosis (6). There were no strict time limits for inclusion based on symptom duration in cases of basilar artery thrombosis. Preexisting intracranial hemorrhage remained an exclusion criterion.
After 9 months of using tPA in place of urokinase, a retrospective review of cases in which tPA had been used for local cerebral fibrinolysis was performed under approval granted by the Human Studies Committee. Symptom duration before treatment, tPA dose administered, heparin dose administered, any other related interventional procedure performed, degree of recanalization achieved, CT evidence of postprocedural intracranial hemorrhage, and in-hospital clinical course were recorded for each case. Data for these tPA-treated patients were compared with data for 20 previously reported cases of basilar artery thrombosis treated with intraarterial urokinase (6).
Results
Four patients with basilar artery thrombosis were treated with intraarterial tPA (Table 1). Symptom duration before treatment ranged from 15 to 67 hours (mean, 38 hours). Total tPA doses ranged from 20 to 50 mg. Intravenous heparin was administered to all patients. Initial heparin boluses ranged from 3000 to 5000 units and subsequent heparin doses during the procedure ranged from 500 to 5000 units, generally to achieve an activated clotting time of two times baseline. Complete basilar artery recanalization was achieved in 75% of patients. Two of four patients had stents placed to augment vertebrobasilar flow at the time of fibrinolysis and one had vertebral artery angioplasty performed elsewhere the day before fibrinolysis. Spontaneous intracranial bleeding complications occurred in three (75%) of four patients treated with tPA. All hemorrhages were larger than 1 cm in diameter. The two patients who had stents placed had parenchymal intracranial hemorrhages remote from the stent locations. The patient with vertebral artery angioplasty without stent placement treated with tPA did not have a hemorrhage. All patients treated with tPA who had intracranial hemorrhages died.
Data for four patients treated for basilar artery thrombosis with intraarterial tPA infusion
In the 20 prior cases of basilar thrombosis treated with intraarterial urokinase, symptom duration before treatment ranged from 1 to 79 hours (mean, 21 hours). Urokinase doses ranged from 250,000 to 1,750,000 units. Intravenous heparin was administered to all patients, with activated clotting times titrated to 1.5 to 2 times baseline during the procedure. Complete basilar artery recanalization was achieved in 50% of patients. No patient had a stent placed. One patient had basilar artery angioplasty. Intracranial bleeding complications occurred in five patients. Of those, two were iatrogenic subarachnoid hemorrhages related to vessel rupture or perforation from microcatheter or angioplasty balloon manipulation, and three (15%) were spontaneous intracerebral hemorrhages no larger than 1 cm in diameter. All patients treated with urokinase who had intracranial hemorrhages died.
Discussion
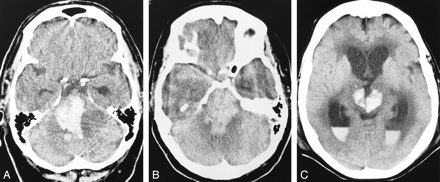
We observed a significantly higher rate of spontaneous cerebral hemorrhage after tPA was infused than after urokinase was infused (Table 2). In addition, in those cases in which there was parenchymal brain hemorrhage after treatment, the extent of hemorrhage observed was greater after treatment with tPA than after treatment with urokinase. Spontaneous cerebral hemorrhages after urokinase treatment tended to be small and were often punctate, whereas hemorrhages after tPA treatment tended to be large or to involve multiple brain stem structures (Fig 1). The increased bleeding observed after treatment with tPA could be attributed to a number of factors.
Comparison of spontaneous intracerebral hemorrhages after local intraarterial fibrinolysis with urokinase and tPA for basilar artery thrombosis
Representative noncontrast brain CT scans in tPA-treated patients with basilar artery thrombosis who had intracerebral hemorrhage after fibrinolysis.
A–C, Case 2 (A), case 3 (B), case 4 (C).
One factor to consider is the dose of tPA used in our series. In the study by Zeumer et al (3) comparing tPA and urokinase for intraarterial fibrinolysis in the setting of acute thromboembolic stroke, a dose of 20 mg tPA was compared with a dose of 750,000 units urokinase administered over a 2-hour time period in 59 patients, 28 of whom had vertebrobasilar clots. There was no time limit for excluding patients with basilar artery thrombosis from treatment, but data on symptom duration before treatment were not reported. All the patients in this series received 5000 units of heparin initially, followed by 1000 units per hour during the procedure. Clot lysis was as effective with tPA as it was with urokinase, and there were no reported symptomatic intracerebral hemorrhages (3). At the time we substituted tPA for urokinase, manufacturers were unable to provide data demonstrating in vivo dose equivalency for tPA and urokinase for intraarterial fibrinolysis for acute thromboembolic stroke or for other intraarterial applications. The National Institute of Neurological Disorders and Stroke (NINDS) trial specified a 0.9 mg/kg dose of tPA (maximum, 100 mg) to be given intravenously for acute stroke (21). It was logical to deliver a dose of tPA locally (intraarterially) that was lower than the dose delivered systemically (intravenously) for stroke, but the lowest efficacious intraarterial dose was not known. We substituted tPA for urokinase at approximately the same ratio used in the comparison study by Zeumer et al (3). Since we had previously delivered up to 1,750,000 units of urokinase over 2 hours for basilar thrombosis rather than the 750,000 units over 2 hours delivered by Zeumer's group, we set our maximum 2-hour intraarterial tPA dose at 50 mg rather than 20 mg. This tPA dose was probably too high. Some investigators suggest that a 10-fold or greater reduction in the doses we used is as efficacious as our doses in achieving clot lysis when tPA is administered intraarterially at the clot surface in peripheral vessels (23).
Another factor to consider is the difference in the mechanisms of action between fibrinolytic agents. For tPA to activate plasminogen, fibrin must be present, but fibrin-bound tPA greatly enhances plasminogen activation. Urokinase activates plasminogen in plasma at a rate similar to activating plasminogen in clot, lacking tPA's fibrin specificity (24). In addition, the molecular weights of the drugs are different (73Da for tPA and 54Da for urokinase), their locations on human genes are different (chromosome 8 for tPA and chromosome 10 for urokinase), and their precursors' characteristics are different (active for tPA and inactive for urokinase) (25). While the two drugs are similar in that they both promote fibrinolysis of intraarterial clots, the differences between them may help to account for the differences in bleeding complication rates observed after fibrinolysis for basilar artery thrombosis.
Of the fibrinolytic agents that are currently available for use, perhaps tPA is not the best choice in this clinical situation. Reteplase (Retevase, Centocor, Inc, Malvern, PA) has a slightly different chemical structure and different binding and half-life characteristics than tPA. The difference between intracranial bleeding complication rates between these two drugs when used for intraarterial cerebral fibrinolysis is unknown, but there may be an advantage for one drug over the other. Streptokinase (Streptase, Astra Pharmaceutical, Westborough, MA) and anistreplase (Eminase, Robbins, Richmond, VA), the only other currently available agents, are susceptible to antistreptokinase antibodies after their initial administration and have not generally been used for treatment of acute stroke (26).
Variables other than the fibrinolytic agent must be considered as potential influences on bleeding rates. The small number of cases in the tPA group could certainly introduce a sampling error, but the exceedingly high rate and extent of intracerebral bleeding in these first few tPA-treated patients is dramatic. Using Fisher's exact test, the rate of spontaneous intracerebral hemorrhage in these two treatment populations is significantly different (P = .035). It would not be prudent to continue using the same protocol and fail to address these initial safety concerns while assuming that the difference in hemorrhagic rates is simply a sampling error or the result of a nonrandomized comparison.
The greater recanalization achieved and the greater use of concomitant vertebrobasilar angioplasty and stent placement in the tPA group relative to the urokinase group could also have had an influence on the hemorrhagic complication and survival rates in the two groups. Improving flow may actually have both positive and negative effects. Because the urokinase group of patients predated the more recent application of coronary microstent technology in the vertebrobasilar system, no patient receiving urokinase had concurrent stent placement. One patient treated with urokinase who repeatedly rethrombosed on plaque underwent basilar artery angioplasty without stent placement using older balloon technology. That patient did not have spontaneous parenchymal hemorrhagic transformation but bled into the subarachnoid space because the vessel ruptured. Another patient in the urokinase group not treated with angioplasty had repeat thrombosis on stenotic plaque and died of the ischemic process, so failure to perform angioplasty to augment flow was also associated with high mortality under certain circumstances. Two patients treated with tPA underwent vertebral artery stent placement and subsequently bled into the brain parenchyma. One patient treated with tPA who underwent vertebral artery angioplasty the day before fibrinolysis had no stent placed, but had no residual stenosis, and did not bleed intracranially. It is possible that concomitant stent placement in the vertebrobasilar system during fibrinolysis for basilar artery thrombosis and successful recanalization further predispose patients to reperfusion hemorrhage in the ischemic territories. On the other hand, basilar artery occlusion does not favor survival. Fibrinolytic agents and heparin together do not always result in recanalization in cases of thrombosis on stenotic plaque. There are new data to suggest that ReoPro (Lilly, Indianapolis, IN), an intravenous antiplatelet drug, at least when given alone in the setting of acute stroke, does not increase the rate of intracranial hemorrhage (27). Perhaps this drug or similar antiplatelet agents should be used rather than stents or additional infusions of a fibrinolytic agent when repeated rethrombosis on plaque during basilar artery fibrinolysis occurs. This issue can only be addressed with further experience treating basilar artery thrombosis.
Systemic heparin anticoagulation was in effect for both groups of patients, and although heparin doses were fairly high and in some cases higher than those used by Zeumer et al (3), heparin anticoagulation was not significantly different in our two groups of patients. Though the heparin doses were similar for our urokinase and tPA groups, it may be that the combined effect of the drugs (heparin and the fibrinolytic agent) is dissimilar. In the data from the first Prolyse in Acute Cerebral Thromboembolism (PROACT) study of intraarterial fibrinolysis for acute middle cerebral artery stroke, heparin facilitated fibrinolysis but increased the hemorrhagic complication rate when given with pro-urokinase, a new fibrinolytic agent not yet approved by the FDA. The heparin bolus dose was adjusted downward from 10,000 units to 2000 units and the maintenance dose was set at 500 units per hour for 2 hours in the second PROACT study. The hemorrhagic rate declined (28, 29). These lower heparin doses may be a reasonable starting point for intraarterial fibrinolysis with tPA.
Symptom duration before treatment was unlimited for both groups and is unlikely to be the major cause of the difference in intracerebral bleeding rates; however, the assumption that unlimited symptom duration before intraarterial fibrinolysis for basilar artery thrombosis is as allowable for tPA treatment as it is for urokinase treatment may be incorrect. Mean symptom duration before treatment was longer for all patients treated with tPA (38 hours) than for all patients treated with urokinase (21 hours), but further examination of the data failed to show that longer symptom duration led to increased intracerebral bleeding. The single tPA-treated patient who did not bleed had a symptom duration of 67 hours, longer than the other tPA-treated patients. The urokinase-treated patients who bled had a mean symptom duration of 7.6 hours, shorter than the mean symptom duration for all urokinase-treated patients. Of the variables that could be altered to lower the risk of hemorrhage after fibrinolysis with tPA, symptom duration is the least desirable to change. Patients with basilar thrombosis often have a delayed diagnosis or present for care relatively late in the course of stroke. Excluding latecomers from treatment for basilar thrombosis leaves such patients a 0% to 20% chance for survival. Every other variable should be investigated further before changes in the protocol are made to exclude patients with basilar artery thrombosis who present outside the 6-hour time frame from treatment.
Our data should caution practitioners that substituting tPA for urokinase at the doses we used is probably not optimal in this clinical situation. We have adjusted our tPA doses considerably downward (to 1.5–15 mg/h) and have further modified the protocol so that reteplase (0.1–1.0 U/h) can be substituted for tPA. These doses were thought to be the most appropriate for further study at a recent meeting (Consensus Conference on Current Strategies for Intracerebral Fibrinolysis for Acute Stroke, Memphis, TN, January 2000). We have also lowered our dose of heparin to that recommended in the PROACT II study (2000-unit bolus plus 500 U/h for 2 hours). We are exercising caution in placing stents to augment vertebrobasilar flow at the same time fibrinolysis for basilar thrombosis is performed, as this may further predispose the ischemic tissues to reperfusion injury. Finally, if the above measures fail to reduce the rate of intracranial bleeding with the available fibrinolytic agents after treatment for basilar thrombosis, some closing of the time window from symptom onset to treatment may be required until urokinase or a drug with a similar safety profile is available.
Conclusion
The cerebral hemorrhage complication rate for intraarterial fibrinolysis with tPA was very high in cases of basilar artery thrombosis at the doses we used. Protocol adjustments should be considered.
Footnotes
↵1 Address reprint requests to DeWitte T. Cross, III, MD.
References
- Received March 16, 2000.
- Copyright © American Society of Neuroradiology