Graphical Abstract
Abstract
BACKGROUND AND PURPOSE: Diffuse gliomas, a heterogeneous group of primary brain tumors, have traditionally been stratified by histology, but recent insights into their molecular features, especially the IDH mutation status, have fundamentally changed their classification and prognosis. Current diagnostic methods, still predominantly relying on invasive biopsy, necessitate the exploration of noninvasive imaging alternatives for glioma characterization.
MATERIALS AND METHODS: In this prospective study, we investigated the utility of the spherical mean technique (SMT) in predicting the IDH status and histologic grade of adult-type diffuse gliomas. Patients with histologically confirmed adult-type diffuse glioma underwent a multiparametric MRI examination using a 3T system, which included a multishell diffusion sequence. Advanced diffusion parameters were obtained using SMT, diffusional kurtosis imaging, and ADC modeling. The diagnostic performance of studied parameters was evaluated by plotting receiver operating characteristic curves with associated area under curve, specificity, and sensitivity values.
RESULTS: A total of 80 patients with a mean age of 48 (SD, 16) years were included in the study. SMT metrics, particularly microscopic fractional anisotropy (μFA), intraneurite voxel fraction, and μFA to the third power (μFA3), demonstrated strong diagnostic performance (all AUC = 0.905, 95% CI, 0.835–0.976; P < .001) in determining IDH status and compared favorably with diffusional kurtosis imaging and ADC models. These parameters also showed a strong predictive capability for tumor grade, with intraneurite voxel fraction and μFA achieving the highest diagnostic accuracy (AUC = 0.937, 95% CI, 0.880–0.993; P < .001). Control analyses on normal-appearing brain tissue confirmed the specificity of these metrics for tumor tissue.
CONCLUSIONS: Our study highlights the potential of SMT for noninvasive characterization of adult-type diffuse gliomas, with a potential to predict IDH status and tumor grade more accurately than traditional ADC metrics. SMT offers a promising addition to the current diagnostic toolkit, enabling more precise preoperative assessments and contributing to personalized treatment planning.
ABBREVIATIONS:
- AK
- axial kurtosis
- AUC
- area under the curve
- CEST
- chemical exchange saturation transfer
- DKI
- diffusional kurtosis imaging
- INVF
- intraneurite voxel fraction
- μFA
- microscopic fractional anisotropy
- MK
- mean kurtosis
- MKT
- mean kurtosis tensor
- RK
- radial kurtosis
- ROC
- receiver operating characteristic
- SMT
- spherical mean technique
- TMD
- transverse microscopic diffusivity
- WHO
- World Health Organization
Diffuse gliomas, a significant portion of primary brain tumors, present challenges in diagnosis and treatment. These tumors have traditionally been classified into low-grade and high-grade gliomas based on histologic features, with the former generally associated with a better prognosis.1 The introduction of molecular markers, notably the isocitrate dehydrogenase (IDH) mutation, has revolutionized this classification, leading to a more nuanced understanding that combines histologic and molecular characteristics for prognosis and treatment-planning. The 2016 World Health Organization (WHO) Classification of the Tumors of the Central Nervous System,2 further refined in 2021, highlights the critical role of IDH mutation status in determining therapeutic approaches and predicting patient outcomes.
Conventional MRI techniques are instrumental in initial glioma assessment. However, their specificity and sensitivity in distinguishing IDH-mutant and IDH-wild-type gliomas are limited, often requiring invasive biopsy for accurate molecular diagnosis.3
Recent advances in diffusion MRI techniques have shown some promise in better characterizing and stratifying gliomas. These techniques have the ability to evaluate tissue microstructure and quantitatively assess the non-Gaussian diffusion behavior of water molecules in tissues.4,5 Among these, the ADC model and diffusional kurtosis imaging (DKI) have been extensively studied,6⇓⇓⇓-10 providing insights into tumor grade and cellular density.
A common pitfall of the above-mentioned models is their inability to disentangle the effects of axonal architecture from the diffusion parameters of the underlying brain tissue.11 This pitfall is a substantial drawback, because neurite microarchitecture varies widely not only between individuals12 but also in different brain regions of a single patient and changes across time.13 The inability to separate these intra- and extra-axonal compartments makes it difficult for traditional diffusion techniques to trace the origin of the pathologic changes in the diffusion patterns. This issue is especially important in the case of diffuse gliomas, in which due to their infiltrative growth, the axonal circuitry remains relatively well-preserved and can obscure potentially important changes due to the presence of underlying pathology.
The spherical mean technique (SMT) emerges as a promising solution to these challenges.14,15 SMT is a recently proposed multicompartmental model that is capable of reconstructing diffusion-based parameters unconfounded by axonal microstructure and capable of separating the diffusion parameters within the intra-axonal and extra-axonal compartments. The output parameters of this method are, therefore, not burdened by intra- and intersubject variability in axonal architecture and are potentially more specific to diffusional alteration happening within the pathologic tissue itself. For example, the fractional anisotropy of the classic diffusion tensor model encodes both the microscopic diffusion process and the fiber-orientation distribution. In contrast, the microscopic fractional anisotropy of SMT is sensitive solely to microscopic diffusion and factors out the intravoxel fiber-orientation distribution. This approach could lead to more specific and clinically relevant markers for noninvasive glioma assessment.15 To date, SMT has been demonstrated to improve lesion detection in the spinal cord of patients with MS;16 however, this is the first study investigating its potential in neuro-oncology.
The hypothesis of this study is that SMT could outperform traditional diffusion MRI metrics in predicting the IDH status and histologic grade of adult-type diffuse gliomas due to its ability to separate diffusion characteristics of intra- and extra-axonal water diffusion. Our aim was to validate the clinical applicability of SMT, potentially enhancing the accuracy of preoperative glioma characterization and supporting more personalized treatment decisions.
MATERIALS AND METHODS
This single-center prospective study was approved by the institutional review board at Military University Hospital, Prague, Czech Republic, and all patients provided written informed consent.
Patient Selection
Adult patients referred to the Military University Hospital, Prague, Czech Republic, between January 2022 and May 2023, with a working diagnosis of adult-type diffuse glioma of any grade have been randomly selected and included in the study. These patients then underwent preoperative MRI and subsequent tumor sampling with histopathologic and molecular/genetic analyses. Patients were excluded if the histopathologic examination confirmed other diagnosis than supratentorial adult-type diffuse glioma, in cases of technically inadequate MRI examination, or in cases with unavailable or incomplete data from genetic analysis.
Data Acquisition
MRI Acquisition.
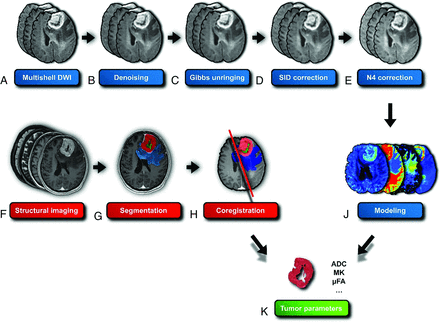
All patients underwent MRI scans immediately (ie, within 24 hours) before biopsy or resection using a 3T MR system GE Discovery 750W (GE Healthcare). The protocol consisted of 2 groups of pulse sequences: 3D structural sequences (T1WI BRAVO, T2WI FLAIR Cube [GE Healthcare], T2WI Cube, 3D T2 susceptibility-weighted angiography [SWAN], and contrast-enhanced T1WI [BRAVO]); and a multishell diffusion sequence (134 non-B0 gradient directions, 11 B0 volumes, and 7 non-B0 b-values). We used the MRtrix gen-scheme (https://github.com/MRtrix3/mrtrix3/blob/master/bin/gen_scheme) for optimal shell sampling.17 For more information about the imaging protocol, see the Online Supplemental Data. The whole processing pipeline for the MRI data is outlined in Fig 1.
Diffusion data-processing pipeline for glioma assessment. Initial multishell DWI (A) is followed by Marchenko-Pastur principal component analysis (MP-PCA) denoising (B) to reduce noise. Subsequent steps include Gibbs ringing artifact removal (C), susceptibility-induced distortion (SID) correction (D), and N4 bias field correction (E) to improve image quality. Structural imaging (F) is used for tumor segmentation (G), which is then coregistered (H) with the diffusion data. Modeling of the diffusion data (J) enables extraction of tumor parameters (K), such as ADC, MK, and μFA. The I letter was intentionally omitted to improve readability.
Histopathologic Data and IDH Status.
Each patient underwent thorough histologic grading and determination of IDH status as part of the standard clinical management protocol. The histologic grade was established using the 5th edition of the World Health Organization Classification of Tumors of the Central Nervous System.2 For further analysis, tumors were split into 2 groups, low-grade glioma (WHO grade 2) and high-grade glioma (WHO grades 3 and 4). The IDH status was determined using next-generation DNA sequencing. Tissue sampling (via biopsy or resection) was performed within 24 hours after the MRI examination.
Tumor Segmentation
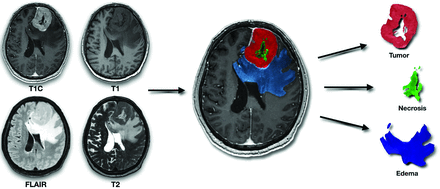
Glioma segmentation was performed using ITK-SNAP (Version 4.0.1; www.itksnap.org), with T1WI, T2WI, FLAIR, and contrast-enhanced T1WI (Fig 1F). An experienced neuroradiologist (V.S.) performed semiautomatic segmentation using the tissue-classification pipeline of ITK-SNAP (Fig 1G). The output segmentation masks included the following: 1) enhancing tumor, 2) nonenhancing tumor, 3) central necrosis, 4) peritumoral T2 hyperintensity (assumed to predominantly represent vasogenic edema if not clearly a part of the nonenhancing tumor), and 5) hemorrhage (to exclude hemorrhagic regions from diffusion and perfusion analysis due to artifacts and possible confounding factors). Tumor enhancement or nonenhacement was verified using motion-corrected subtraction of pre- and postcontrast T1WI. The presence of enhancing tumor, central necrosis, T2 FLAIR mismatch, and hemorrhage was used in the subsequent analysis, with respective binary variables indicating the presence or absence of each feature. Illustration of tumor segmentation and generation of tumoral masks is shown in Fig 2.
Illustration of tumor segmentation and generation of tumoral masks. The figure demonstrates the segmentation of gliomas using 4 image sequences (from left to right and top to bottom: contrast-enhanced T1-weighted, T1-weighted, FLAIR, and T2-weighted). These masks are subsequently coregistered with diffusion data sets.
Extraction of Neuroimaging Parameters
Diffusion Analysis.
Diffusion data (Fig 1A) were preprocessed using FSL (Version 6.0.6; http://www.fmrib.ox.ac.uk/fsl), MRtrix3 (Version 3.0.4; https://www.mrtrix.org/2022/12/16/mrtrix-3-0-4-release/), and Advanced Normalization Tools (Version 2.5.00, ANTs; http://stnava.github.io/ANTs/) toolkits. Preprocessing included the following: 1) Marchenko-Pastur principal component analysis denoising (Fig 1B),18 2) Gibbs ringing removal (Fig 1C),19 3) motion and eddy current distortion correction,20 4) susceptibility-induced distortion correction (Fig 1D),21 and 5) N4 bias field correction (Fig 1E).22
DKI, ADC, and DTI modeling (Fig 1J) were performed using the Diffusion Imaging in Python (DIPY) toolbox (Version 1.7.0; https://dipy.org/), with ADC and DTI using only b = 0 and b = 1000 shells. SMT maps were reconstructed using the original code introduced by Kaden et al,14,15 available at github.com/ekaden/smt. Comparison of maps showing ADC, DTI, DKI, and SMT models in a selected tumor sample are shown in the Online Supplemental Data.
Diffusion Parameter Extraction.
After coregistering the structural images with their respective tumor segmentation masks to the diffusion space using ITK-SNAP (Fig 1H), we overlaid the tumor masks onto the diffusion maps (enhancing tumor mask in case of enhancing tumors, nonenhancing tumor mask in case of nonenhancing tumors). Then, we extracted the intensity values for each voxel within the tumor area. The acquired data sets for each patient were converted into a NumPy array I (Version 1.25.0; https://numpy.org/doc/stable/release/1.25.0-notes.html; Python 3.10), and the mean values for each parameter were extracted using the SciPy library (Version 1.10; https://docs.scipy.org/doc/scipy-1.10.1/; Python 3.10). In total, 17 diffusion parameters were extracted from the ADC, DTI, DKI, and SMT models (Fig 1K), all using the same approach.
Statistical Analysis
Prediction of IDH Status and Histologic Grade.
The primary objective was to assess the association among IDH status, glioma grade, and the SMT, DKI, and ADC parameters. Receiver operating characteristic (ROC) curves were generated using the pROC library (https://bmcbioinformatics.biomedcentral.com/articles/10.1186/1471-2105-12-77) to assess the diagnostic performance of the models for predicting IDH status and glioma grade. Optimal decision thresholds were identified by area under the curve (AUC) and Youden J values for sensitivity and specificity. To quantify the strength and direction of the relationships between the studied diffusion metrics and both IDH and glioma grades, we created a correlation matrix by calculating Pearson coefficients using the gplots (https://www.rdocumentation.org/packages/gplots/versions/3.1.3.1) and corrplot (https://www.rdocumentation.org/packages/corrplot/versions/0.92) libraries. Statistical analyses were conducted in R statistical and computing software (Version 4.0.3; http://www.r-project.org/) with a significance level of .05.
RESULTS
Patient Characteristics
Among 108 patients with a working diagnosis of adult-type diffuse glioma, 28 were excluded for malignancies other than adult-type diffuse glioma. No patients were excluded for technical reasons. Therefore, the final study cohort consisted of 80 patients with histologically confirmed adult-type diffuse glioma, with a mean age of 48 (SD, 16) years (range, 21–83 years). Most patients were men (n = 49, 61%). Regarding the histology and genetic markers, 43 subjects (54%) had a high-grade glioma, and 46 (58%) were positive for IDH mutation. Postcontrast enhancement was observed in 46 patients (58%), while necrosis was noted in 31 (39%). T2 FLAIR mismatch was observed in 6 patients (8%). Hemorrhage was present in 24 patients (30%). Finally, elevated relative CBF was present in 44 patients (55%). Detailed data regarding the included cohort are shown in Table 1.
Patient characteristicsa
Prediction of IDH Status
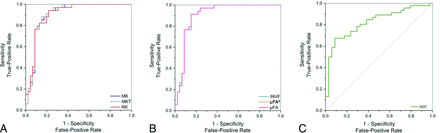
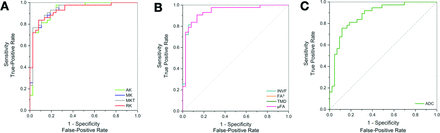
We explored the discriminative power of the diffusion metrics in relation to IDH status. Our findings demonstrated significant differences in the SMT characteristics between IDH-mutant and IDH-wild-type gliomas. The SMT metrics with the highest diagnostic performance in the determination of IDH status were microscopic fractional anisotropy (μFA), microscopic fractional anisotropy to the third power (μFA3), and intraneurite volume fraction (INVF), all with AUC = 0.91 (95% CI, 0.84–0.98; P < .001), followed by transverse microscopic diffusivity (TMD), with AUC = 0.87 (95% CI, 0.79–0.95; P < .001). Among the non-SMT metrics, the best-performing was mean kurtosis tensor (MKT), with AUC = 0.91 (95% CI, 0.84–0.98; P < .001) and mean kurtosis (MK) and radial kurtosis (RK), both with AUC = 0.90 (95% CI, 0.83–0.97; P < .001). For reference, ADC showed AUC = 0.82 (95% CI, 0.73–0.92; P < .001).
The investigated nondiffusion imaging parameter with the highest diagnostic performance was the presence of necrosis (AUC = 0.85, 95% CI, 0.77–0.94; P < .001). For more detailed results, please see Table 2 and Fig 3.
ROC curves for selected parameters illustrating the diagnostic accuracy in determining IDH status. The curves compare DKI (A), SMT parameters (B), and the standard clinically used ADC method (C), with an emphasis on parameters with AUC values of >0.90.
Comparative diagnostic accuracy of investigated parameters differentiating IDH-mutant from IDH-wild-type gliomasa
Prediction of Histologic Grade
Next, we examined the predictive power of the investigated diffusion metrics toward glioma grade. The best diffusion-based predictors of grade in our study were INVF, μFA, μFA,3 all with AUC = 0.94 (95% CI, 0.88–0.99; P < .001). Among the highest-scoring non-SMT metrics, MKT performed with the best with AUC = 0.94 (95% CI, 0.88–0.99; P < .001), along with RK and MK, both with AUC = 0.93 (95% CI, 0.88–1.00; P < .001) and axial kurtosis (AK) with AUC = 0.93 (95% CI, 0.87–1.00; P < .001). The ADC model had AUC = 0.88 (95% CI, 0.80–0.96; P < .001). Detailed results regarding the prediction of histologic grade are presented in Table 3 and Fig 4.
ROC curves for selected parameters illustrating the diagnostic accuracy in determining glioma grade.The curves compare DKI (A), and SMT (B), parameters with the standard clinically used ADC method (C), with an emphasis on parameters with AUC values of 0.90.
Comparative diagnostic accuracy of investigated parameters differentiating glioma gradesa
To provide a comprehensive overview of the complex relationships among various advanced diffusion parameters, we have included a detailed correlation matrix (Online Supplemental Data).
DISCUSSION
In this study, we investigated for the first time the potential of the SMT in predicting 2 crucial factors in glioma characterization: tumor grade and IDH status. To evaluate its clinical potential, we compared the diagnostic performance of SMT with other, more established diffusion MR techniques. Our findings suggest that SMT has significant potential for characterizing adult-type gliomas. Notably, several parameters, specifically µFA, µFA3, and INVF demonstrated considerable predictive capabilities, each achieving a high AUC of 0.94 in predicting glioma grade and 0.91 for IDH status, indicating their potential clinical utility (P < .001).
In particular, our results emphasize the utility of μFA, a metric designed to quantify the degree of directionality of water diffusion independent of axonal orientation dispersion. μFA provides insights into the cellular and extracellular architecture of the tissue, unconfounded by the surrounding axonal microstructure.14,15 In the context of gliomas, IDH mutations are known to induce distinct biologic alterations, including changes in cellular density, morphology, and the organization of the extracellular matrix.23,24 These changes in the glioma microenvironment result in decreased directionality and lower coherence of water diffusion patterns, with lower μFA values being associated with the presence of an IDH mutation. Our findings demonstrate that μFA is particularly adept at detecting these variations, reinforcing its potential as a diagnostic tool in assessing glioma characteristics.
Another notable finding is the robust relationship between INVF and IDH status. INVF is designed to quantify the fraction of the voxel occupied by intraneurite space, essentially reflecting the density of neurite-like structures within the tumor microenvironment. However, the underlying reasons for its significant association with IDH status remain unclear. A possible explanation could be that tissue changes, more commonly observed in aggressive glioma variants, are perceived to have axon-like diffusion properties by the SMT model, causing the strong correlation. Alternatively, the association may be a result of modeling constraints imposed on the INVF parameter by the SMT model. Microstructural models, including SMT, typically assume that bodily tissues can be described as finite, often a small sum of Gaussian compartments.25 This approach, while necessary for practical reasons (eg, computational time), oversimplifies the complex nature of brain tissue and its intra- and extracellular compartments. Consequently, this simplification may result in the decreased specificity of parameters attempting to characterize portions of these compartments.
The study also revealed a strong predictive performance of SMT metrics in the evaluation of glioma grade. These results further confirm that diffusion parameters may reflect the underlying pathologic processes of gliomas, such as cellularity,10 which are critical determinants of tumor grade.
Our findings regarding the utility of ADC and DKI in glioma characterization align with previous studies,11,26 confirming the added utility of advanced diffusion imaging in the characterization of brain gliomas.
Despite these promising findings, our study has limitations. The sample size, though adequate for initial exploration, warrants expansion in future studies to enhance the generalizability of the results and provide clearer separation between the diagnostic performances of the investigated predictive features. Another potential limitation is the relatively long acquisition duration of the used diffusion sequence. While still within the limits of clinical feasibility, an addition of a 9-minute pulse sequence may not be feasible for many centers. However, our study design intentionally included a robust diffusion sequence, and SMT parameters can be reconstructed using only 2 nonzero b-values, instead of 6 used in our study. The reduction of number of b-values in combination with SNR27 and angular resolution28 enhancing algorithms can significantly reduce scanning times to more manageable clinical durations of about 2−3 minutes.
Integrating SMT with other emerging imaging modalities, such as amino acid PET, chemical exchange saturation transfer (CEST) MRI, and MR spectroscopy, could further refine the noninvasive characterization of gliomas. The inclusion of CEST MRI, which offers surrogate biomarkers for amides and amines crucial in glioma subtyping, could provide a valuable enhancement to our imaging arsenal, as evidenced by the work of Mancini et al.29 Similarly, the application of machine-learning algorithms,30,31 other advanced diffusion techniques (eg, neurite orientation dispersion and density imaging [NODDI]),32 or higher-field-strength MR spectroscopy could provide a more detailed metabolic profile of gliomas, enhancing our understanding of their unique characteristics and aiding in more accurate grading.
Investigating the longitudinal changes in SMT parameters during treatment and follow-up could provide valuable information on tumor response and progression. This would be particularly useful in assessing the efficacy of targeted therapies that might influence tumor microstructure in a manner different from traditional chemoradiation.
Finally, the development of automated, artificial intelligence–driven algorithms for SMT parameter analysis could facilitate the translation of this technique into routine research or even clinical practice, making it more accessible for widespread use.
Conclusions
Our study underscores the potential of SMT as a significant noninvasive tool for predicting IDH status and tumor grading in adult-type diffuse gliomas, enhancing preoperative tumor characterization and potentially influencing treatment decisions and outcomes. The diagnostic performance of SMT parameters, despite certain limitations, offers promising avenues for advancing glioma diagnostics. Future directions should focus on refining microstructural models to better understand the biologic underpinnings of SMT measurements, expanding sample sizes, and incorporating advanced imaging modalities to enrich the noninvasive assessment of gliomas. Additionally, investigating the changes in the model parameters during treatment could offer insights into tumor response and progression, thereby contributing to more tailored and effective therapeutic strategies.
Footnotes
This research was supported by Charles University, project GA UK No. 222623. Institutional support MO1012 was by the Ministry of Defense of the Czech Republic, and Cooperation Neuroscience was by Charles University. Research by M.N. was partially supported by the institutional resources of Czech Technical University in Prague and Karolinska Institute.
The funders of the study had no role in the study design or the collection, analysis, and interpretation of data, writing of the report, or decision to submit the manuscript for publication.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received May 14, 2024.
- Accepted after revision July 10, 2024.
- © 2025 by American Journal of Neuroradiology