Abstract
Summary: We present serial MR perfusion and spectroscopic findings of a pathologically proved low grade glioma, which evolved into glioblastoma multiforme in 2 years in a 24-year-old man. The initial MR imaging studies, including enhanced conventional T1-weighted and perfusion imaging, were characteristic of a benign glioma with the only exception being that multi-voxel proton MR spectroscopy showed malignant features with a high choline:phosphocreatine ratio. Postoperative follow-up MR imaging revealed findings consistent with malignant glioma, with increased angiogenesis on perfusion images and heterogeneous enhancement on contrast-enhanced T1-weighted images that were further confirmed by second surgery. We suggest conducting close MR imaging follow-up of patients with glioma who have discrepant MR spectroscopic and perfusion results after treatment.
The prognostic significance of histopathologic and molecular biologic factors in brain tumors that can be partially revealed by MR perfusion and spectroscopic imaging is of great interest because these factors are potentially able to guide clinical management decisions. In this report, we present serial MR perfusion and spectroscopic findings of a pathologically proved low grade glioma, which evolved into glioblastoma multiforme in 2 years in a 24-year-old patient. The significance of initial discrepant MR spectroscopic and perfusion imaging results is discussed.
Case Report
A 24-year-old man with a first seizure attack presented to our hospital in April 1998. A cranial CT study showed a hypoattenuated mass in the left temporal lobe. An MR imaging examination was subsequently performed on a 1.5-T system, including unenhanced axial view T1-weighted spin-echo (700/14/1 [TR/TE/number of excitations]), T2-weighted fast spin-echo (4000/99/1; echo train length, 7), and fluid-attenuated inversion recovery (9000/110; inversion time, 2500 ms) imaging of the brain. Multi-voxel proton MR spectra were then acquired by using chemical shift imaging based on the point-resolved spectroscopic technique (TE, 270 ms; subvoxel size, 1 × 1 × 3 cm3). Dynamic susceptibility contrast-enhanced perfusion-weighted MR imaging was performed by using gradient-echo echo-planar imaging (TE, 44 ms; field of view, 230 mm; matrix, 102 × 128; section thickness, 5 mm; time interval of 1 s with 75 consecutive measurements and six sections in each measurement) during the first-pass transit of IV bolus injection of contrast material. Maps of relative cerebral blood volume (rCBV) were generated on a pixel-by-pixel basis through numerical integration of the contrast agent concentration time curves. Finally, contrast-enhanced axial view T1-weighted images were obtained.
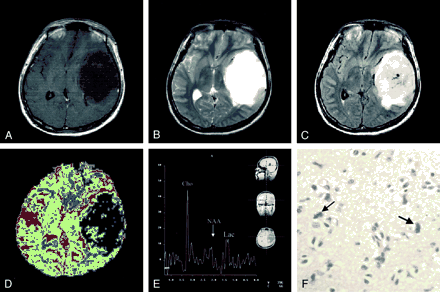
The MR imaging and spectroscopic findings are shown in Figure 1. There was no enhancement on the contrast-enhanced T1-weighted images (Fig 1A). No obvious white matter edema was observed on T2-weighted or fluid-attenuated inversion recovery images (Fig 1B and C). The rCBV was abnormally lower in the lesion than in the contralateral homologous normal hemisphere (Fig 1D). MR spectroscopy showed markedly increased choline, whereas N-acetylaspartate was found to decrease to almost zero (Fig 1E). Note that except for the spectroscopic results that suggested tumor malignancy (1), all other MR imaging findings were consistent with those of benign glioma. After craniotomy, the left temporal lobe tumor was proved pathologically to be a low grade glioma (Fig 1F). Radiation therapy with a total dose of 66.6 Gy was delivered during a 6-week interval after the patient’s discharge.
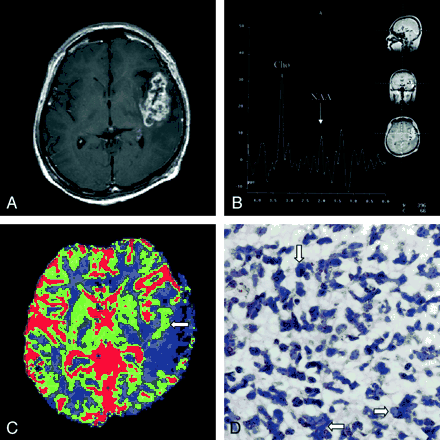
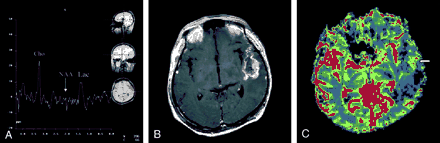
The post-hospital course was uneventful until June 2000, when the patient developed symptoms of headache and vomiting. He was sent to the emergency department, where CT of the brain revealed brain edema. Supportive treatment with steroid infusion was administered. Symptoms of headache and nausea improved. An MR imaging examination was performed by using the same protocol as stated previously, showing a recurrent tumor in the left temporal lobe. Choline concentration, as revealed by proton MR spectroscopy, remained high, and N-acetylaspartate was hardly found (Fig 2B), again suggesting malignant cerebral glioma. Contrast-enhanced T1-weighted imaging showed enhancement (Fig 2A), consistent with the spectroscopic findings associated with malignant brain tumor. Moreover, increased rCBV began to appear in lesion tissue, compared with the previously low rCBV shown by mapping (Fig 2C). A second surgical intervention was performed, and the histopathologic examination revealed glioblastoma multiforme (Fig 2D). After being discharged from the hospital, the patient’s health condition was stable until January 2001, during which the patient developed dizziness, vomiting, diplopia, and aphasia. In addition to conservative treatments, including glycerol and steroid therapy, palliative radiation therapy was administered with a total dose of 59.4 Gy during another 7-week interval. Follow-up CT of the brain revealed brain swelling and recurrence of tumor in the left temporal lobe. A third MR spectroscopic study showed a high choline concentration, and no N-acetylaspartate peak was detectable (Fig 3A). Contrast-enhanced T1-weighted imaging showed heterogeneous enhancement of the left temporal lobe tumor (Fig 3B). The rCBV map showed increased blood volume in tumor territory in contrast to previous perfusion examinations (Fig 3C). In September 2001, the patient died as a result of cardiovascular failure after the occurrence of increased intracranial pressure presenting as intractable nausea, vomiting, and headache, with a survival time of 3 years and 5 months since the first operation.
Discussion
Traditionally, a combination of T1- and T2-weighted MR imaging has been used for the diagnosis of cerebral glioma (2); however, difficulties are encountered when discrepancies exist between MR imaging findings and pathologic reports (3). Recent developments in new MR imaging techniques have allowed complementary diagnostic information to be provided in vivo before surgical intervention. For instance, the results presented by Aronen et al (4) suggest that rCBV maps obtained by dynamic susceptibility contrast-enhanced MR imaging help grade cerebral gliomas and depict areas of varying hemodynamic activities within the tumors and surrounding tissue. On the other hand, proton MR spectroscopy may assist discrimination of high grade from low grade gliomas in some difficult cases in which histologic uncertainties exist between anaplastic astrocytomas and low grade oligodendrogliomas (5). In addition to the individual diagnostic capability and the relationship between these MR indices and pathologic findings, it is worthwhile to assess their prognostic ability to guide clinical management decisions.
In our case, both MR imaging and histologic examination of the tumor in the initial stage indicated that the glioma was of low grade, with the only exception being the results of proton MR spectroscopy, which suggested malignancy. In particular, the corresponding rCBV values were abnormally low in a large portion of the tumor territory compared with the contralateral homologous region (Fig 1D), which implied absence of aggressive angiogenesis and a possible benign tumor (3), findings that are consistent with those of conventional MR imaging. MR spectroscopic results, however, indicated an existence of highly active membrane turnover by showing a substantially increased choline concentration and reduced N-acetylaspartate (Fig 1E) (1). Recurrence of tumor in the form of malignant glioblastoma multiforme, proved both radiologically (Fig 2) and histologically, was found 2 years later at approximately the same location, strongly supporting the first MR spectroscopic findings of early stage tumor malignancy. Furthermore, throughout the three MR spectroscopic examinations, the choline:creatine ratio measured from the tumor remained extremely high (5.63, 6.35, and 7.18 respectively). On the other hand, although mild blood volume increase was observed during follow-up perfusion examinations, three MR perfusion images showed lower rCBV in the lesion territory compared with the contralateral homologous normal brain tissue. Therefore, we postulate that proton MR spectroscopy might have better prognostic value than the rCBV mapping in cases of cerebral glioma. According to previous studies, it is common that a tumor mutates between benign and malignant stages in the process of development or therapy (6, 7) and malignant transformation of a benign tumor shows serial spectral changes (1). Tedeschi et al (8) proposed that progressive tumors show a choline increase between serial studies of more than 45%, whereas stable cases show an elevation of less than 35% or even a decreased choline signal intensity. Our case is contradictory to the study presented by Wald et al in that the choline:creatine ratio of the tumor remained high throughout the patient’s course from benign to malignant stages. Because proliferation of tumor cells recruits vessel network to provide nutrition for further growth, elevated choline concentration, indicating high cellularity, is usually accompanied by increased rCBV; however, the interaction may not be well established during the early stage. Thus, we speculate that an abnormally high choline concentration with low rCBV is an early sign of malignant transformation. Also, because histologic confirmation of the exact location and cell grading of the tissues sampled from a large tumor for pathologic diagnosis are not easy if preoperative stereotactic biopsy is not performed, the validity of the initial pathologic diagnosis of low grade glioma based on piece-by-piece surgical removal of specimens may be limited, even though the general condition after the first operation was good.
Another interesting issue was radiation therapy as a contributing factor of the malignant transformation of the low grade glioma in our case. Radiation-induced malignant glioma could be observed in patients who survived other brain tumors during 10- to 15-year follow-up (9). Malignant transformation of low grade glioma has not been extensively studied. Dirks et al (10) reported the cases of six children with malignant transformation of low grade astrocytomas after low dose radiation therapy (50–52 Gy) in an interval from 2 to 10 years (mean, 6.4 years). In our case, glioblastoma multiforme was diagnosed 2 years after the completion of initial radiation therapy (66.6 Gy). The role of a higher dose of radiation on the malignant transformation of low grade glioma requires further investigation.
We suggest that a multi-technique MR imaging examination can be an effective tool for the early stage prognosis of cerebral gliomas. MR spectroscopy reveals the metabolic status and is complementary to rCBV mapping, which indicates hemodynamic activity. Both of the above techniques assist radiologic diagnosis by providing information supplementary to conventional MR imaging methods. Moreover, these diagnostic modalities can be applied in a spatially registered manner and therefore are not limited by finite sample size, as is the case with surgical biopsy and pathologic examination. Although the relative efficacy of MR spectroscopy, perfusion-weighted imaging, and diffusion-weighted imaging (11) for a larger patient population remains to be investigated, our case report indicates that the possibility of tumor malignancy, as suggested by early stage proton MR spectroscopy, should not be overlooked.
Initial MR imaging examination performed in April 1998.
A, No enhancement of the hypointense tumor mass in the left temporal lobe was revealed by contrast-enhanced T1-weighted images.
B, No obvious associated white matter edema was observed on T2-weighted images.
C, No obvious associated white matter edema was observed on fluid-attenuated inversion recovery images.
D, rCBV map showed abnormally lower blood volume in the lesion than in the contralateral homologous normal hemisphere.
E, Corresponding MR spectroscopic findings showed markedly increased choline (Cho), whereas N-acetylaspartate (NAA) was found to decrease to almost zero. Lac, lactate.
F, Photomicrograph showed some tumor cells of astrocytic origin, with moderate pleomorphism and chromatin attenuation (hematoxylin and eosin; original magnification, ×400).
Second MR imaging examination performed in June 2000, using same protocol as that used for first examination.
A, Contrast-enhanced T1-weighted images showed an enhancing lesion in the previous surgical bed over the left temporal lobe.
B, MR spectral sampling from the abnormal enhancing lesion showed a high choline (Cho) concentration and exceedingly low N-acetylaspartate (NAA).
C, rCBV map showed slightly increased blood volume in the medial aspect of tumor territory (arrow), compared with the unusual absence in the first examination. Nevertheless, the rCBV remained largely lower in the lesion than in the contralateral homologous normal hemisphere.
D, Photomicrograph, obtained 27 months after the first histologic study, showed malignant undifferentiated tumor cells with marked cytoplasmic and nuclear pleomorphism (arrows). Packed large tumor cells with densely hyperchromatic nuclei were noted (hematoxylin and eosin; original magnification, ×400). The tumor also showed areas of necrosis (not shown).
Third MR spectroscopic study performed in January 2001.
A, High choline (Cho) concentration and hardly detectable N-acetylaspartate (NAA) peak could be seen. Lac, lactate.
B, Contrast-enhanced T1-weighted images showed heterogeneous enhancement of the left temporal lobe tumor.
C, In contrast to the low blood volume in lesion revealed by the first perfusion study 3 years previously, the rCBV map began to show increased blood volume in the superficial region of the tumor area (arrow).
References
- Received April 15, 2002.
- Accepted after revision June 11, 2002.
- Copyright © American Society of Neuroradiology