Abstract
BACKGROUND AND PURPOSE: Verbal memory decline is a common complaint of patients with severe asymptomatic stenosis of the internal carotid artery (aICS). Previous publications explored the associations between verbal memory decline and altered functional connectivity (FC) after aICS. Patients with severe aICS may show reduced perfusion in the ipsilateral territory and redistribution of cerebral blood flow to compensate for the deficient regions, including expansion of the posterior and contralateral ICA territories via the circle of Willis. However, aICS-related FC changes in anterior and posterior territories and the impact of the sides of stenosis were less explored. This study aims to investigate the altered FC in anterior and posterior circulation territories of patients with left or right unilateral aICS and its association with verbal memory decline.
MATERIALS AND METHODS: We enrolled 15 healthy controls (HCs), 22 patients with left aICS (aICSL), and 33 patients with right aICS (aICSR) to receive fMRI, Mini-Mental State Examination (MMSE), the Digit Span Test (DST), and the 12-item Chinese version of Verbal Learning Tests. We selected brain regions associated with verbal memory within anterior and posterior circulation territories. Territory-related FC alterations and verbal memory decline were identified by comparing the aICSL and aICSR groups with HC groups (P < .05, corrected for multiple comparisons), respectively. Furthermore, the association between altered FC and verbal memory decline was tested with the Pearson correlation analysis.
RESULTS: Compared with HCs, patients with aICSL or aICSR had significant impairment in delayed recall of verbal memory. Decline in delayed recall of verbal memory was significantly associated with altered FC between the right cerebellum and right middle temporal pole in the posterior circulation territory (r = 0.40, P = .03) in the aICSR group and was significantly associated with altered FC between the right superior medial frontal gyrus and left lingual gyrus in the anterior circulation territory (r = 0.56, P = .01) in the aICSL group.
CONCLUSIONS: Patients with aICSL and aICSR showed different patterns of FC alterations in both anterior and posterior circulation territories, which suggests that the side of aICS influences the compensatory mechanism for decline in delayed recall of verbal memory.
ABBREVIATIONS:
- AAL
- Automatic Anatomical Labeling Atlas
- aICS
- asymptomatic internal carotid artery stenosis
- aICSL
- left-side asymptomatic internal carotid artery stenosis
- aICSR
- right-side asymptomatic internal carotid artery stenosis
- BOLD
- blood oxygen level–dependent
- DST
- Digit Span Test
- FC
- functional connectivity
- FDR
- false discovery rate
- HC
- healthy control
- MMSE
- Mini-Mental State Examination
- rs-fMRI
- resting-state fMRI
- SPM12
- Statistical Parametric Mapping 12
- VCID
- vascular contributions to cognitive impairment and dementia
SUMMARY
PREVIOUS LITERATURE
Previous literatures have reported the verbal memory decline in asymptomatic internal carotid artery stenosis (aICS). However, the functional connectivity (FC) alterations and associated compensatory mechanisms induced by the brain hypoperfusion in the unilateral hemisphere were less explored. In this study, we aim to investigate the impact of two key factors, including the circulation territories (anterior and posterior circulation) and the laterality of aICS (left and right hemisphere), on the FC alterations and verbal memory decline. The difference of compensatory mechanisms between left aICS (aICSL) and right aICS (aICSR) groups was investigated.
KEY FINDINGS
Decline in delayed recall of verbal memory was significantly associated with alterations of FC within the posterior circulation territory in the aICSR group and was significantly associated with alterations of FC within the anterior circulation territory in the aICSL group.
KNOWLEDGE ADVANCEMENT
The differences in compensatory mechanisms reflecting on FC alterations between the aICSL and aICSR groups may be associated with the lateralization of verbal memory (ie, left hemisphere–dominant). We provided neuroimaging evidence suggesting the influences of the side in which aICS occurs on the decline in delayed recall of verbal memory.
Asymptomatic internal carotid artery stenosis (aICS) is defined as the presence of ICA stenosis without a history of ischemic events.1 With advances in contemporary medical management, the estimated occurrence rate of ipsilateral acute ischemic stroke in patients with aICS has dropped to 4.7% over 5 years.2 However, verbal memory decline is one of the major cognitive complications for patients with aICS.3⇓⇓⇓-7
Patients with severe aICS may show reduced perfusion in the ipsilateral territory and redistribution of cerebral blood flow to compensate for the deficient regions, including expansion of the posterior and contralateral ICA territories via the circle of Willis.8⇓-10 Previous studies have reported an association between brain perfusion and functional connectivity (FC),11 which is further correlated with verbal memory function.12 He et al13 reported an association between FC disruption in left aICS and impairment of short-term memory. However, further investigation is needed to determine the FC alterations and verbal memory impairment of patients with aICS.
Performing verbal memory tasks can activate the superior medial frontal gyrus, middle temporal pole, lingual gyrus, cerebellum VIII, and vermis VIII.14⇓-16 The superior medial frontal gyrus, which is involved in attention, is in charge of memory storage;17 the middle temporal pole and lingual gyrus, which process visual stimuli and text, are responsible for memory encoding;18,19 cerebellum VIII and vermis VIII are involved in the process of memory manipulating.20 Previous resting-state functional MR imaging (rs-fMRI) studies have proposed activation alterations in these regions after aICS.14,15 Accordingly, studying the alterations in FC among these regions may provide potential biomarkers for verbal memory decline after aICS.
Language is one of the main components of verbal memory.21 Functional brain areas in the language network are mainly in the left hemisphere,22 and the left superior temporal gyrus is one of the key areas for verbal memory.23,24 Reduced perfusion in the left or right hemisphere may therefore lead to different degrees of verbal memory decline. However, the impact of the side of aICS on verbal memory decline is still unclear. Furthermore, FC in homologous brain regions could be altered by acute hypoxia. Guo et al25 reported increased FC in the contralesional temporal gyrus and inferior frontal gyrus in patients with poststroke aphasia. However, the functional alterations due to left and right unilateral aICS have received less attention.
Considering the lateralization of brain functions, we hypothesized that stenosis in the left and right ICA may cause verbal memory decline and FC alterations with different compensatory mechanisms. This study aimed to evaluate verbal memory impairment and identify FC alterations in anterior and posterior circulation territories in patients with left or right unilateral aICS.
MATERIALS AND METHODS
Participants
We prospectively enrolled 22 patients with asymptomatic stenosis in the left ICA (aICSL), 33 patients with asymptomatic stenosis in the right ICA (aICSR), and 15 age- and sex-matched healthy controls (HCs). The inclusion criteria were as follows: 1) diagnosed with severe aICS (defined as stenosis of lumen diameter greater than 50%), 2) had T1-weighted images and functional MR images, and 3) had MR images free from old infarcts or other structural lesions. The exclusion criteria were as follows: 1) history of transient ischemic attack or ischemic stroke, 2) functional disability (modified Rankin Scale score >2), 3) clinical diagnosis of dementia according to Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, or 4) other major neurologic and psychological disorder (such as Parkinsonism, depression, generalized anxiety disorder) or malignancy. Neuropsychological tests, including the 12-item Chinese version of immediate and delayed recall of Verbal Learning Tests, were used to evaluate working verbal memory and short-term verbal memory performance, respectively.26 In the 12-item Chinese Version Verbal Learning Test, participants were asked to memorize 12 items. First, a neuropsychologist read the 12 items 4 times. The participant then had 5 recall trials 30 seconds later. The sum of item numbers recalled in each trial was indicated as immediate recall performance. Afterward, the participant was requested to recall the items once again 10 minutes later, the sum of which was used to determine the score of delayed recall. The comprehensive cognitive function of each case was assessed by using Mini-Mental State Examination (MMSE). The Digit Span Tests (DST), including forward and reverse tests, were employed to evaluate attentional capacity and working memory, especially for number sequences. This study was approved by the Institutional Review Board of the Taipei Veterans General Hospital (VGHIRB No. 2012–01-016AC; 2015–11-006C; 2020–02-017A). All the participants provided written informed consent before participating in this study.
MR Imaging Acquisition
MR imaging data, including 3D T1WI data, 3D FLAIR data, and blood oxygen level–dependent (BOLD) fMRI data, were collected by using a 3T Discovery 750 MR scanner (GE Healthcare). The 3D T1WI data were acquired by using a axial MRI 3D brain volume (BRAVO) sequence (TR/TE: 12.2/5.2 ms, flip angle: 12°, voxel size: 1 × 1 × 1 mm3, and field of view: 256 × 256 mm2). Rs-fMRI data were acquired by using the gradient-echo echo-planar imaging sequence (TR/TE: 3000/30 ms, flip angle: 90°, thickness: 3 mm, field of view: 222 × 222 mm2, and 124 repetitions). During BOLD fMRI scanning, the participants were instructed to remain relaxed and awake and keep their eyes open. The 3D FLAIR images were acquired with TR of 9000 ms, TE of 143.9 ms, flip angle of 110°, voxel size of 0.5 × 0.5 × 0.5 mm3, and field of view of 256 × 256 mm2. This study adopted the Fazekas scores to evaluate white matter hyperintensities on FLAIR images.27
Image Preprocessing
The fMRI data were processed following the standard procedure by using Statistical Parametric Mapping 12 (SPM12).28 After removing the first six time points, we performed corrections for slice timing, realignment, coregistration of T1WI to BOLD images, spatial normalization, and spatial smoothing with an 8-mm full width at half maximum Gaussian kernel and regressed out the confounding effects of motion parameters and signals from white matter and CSF. In this study, all the participants showed minor head motion during the fMRI examination and were all included in the subsequent analyses.
Analysis of FC Associated with Verbal Memory
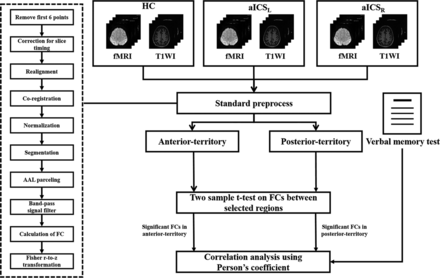
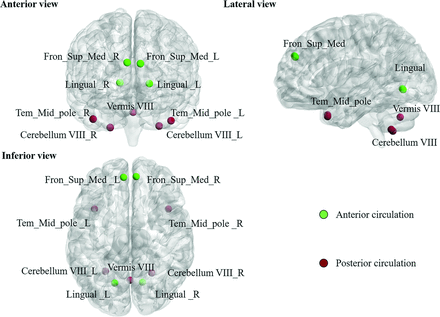
In this study, the targeted regions, including the superior medial frontal gyrus, lingual gyrus, middle temporal pole, cerebellum VIII, and vermis VIII, were parceled based on the Automatic Anatomical Labeling atlas.29 The average BOLD signals of these targeted regions were bandpass-filtered with the frequency band of 0.01–0.1 Hz. The FC was estimated by calculating the Pearson correlation coefficients between each pair of targeted regions in the anterior and posterior circulation territories, followed by Fisher r-to-z transformation (Fig 1). The 4 × 4 FC matrix among 4 areas, including the bilateral superior medial frontal and lingual gyri, was used to measure the FC within the anterior circulation. The 5 × 5 FC matrix among 5 areas, including vermis 8, the bilateral middle temporal pole, and cerebellum VIII, was used to assess FC in areas in the posterior circulation territory (Fig 2).
Flow chart for the study.
The 9 brain regions were parceled based on the automated anatomical labeling atlas with 116 areas (AAL 116) and highlighted in the following analyses. The green nodes indicate brain regions in the anterior circulation territory; the wine-colored nodes indicate brain regions in the posterior circulation territory.
Furthermore, because the language network is one of the main components of verbal memory, we also calculated the 6 × 6 FC matrix among primary language regions, including Broca, Geschwind, and Wernicke areas and corresponding regions in the bilateral hemispheres.
Statistical Analyses
To investigate the altered FC within the anterior circulation territory, posterior circulation territory, and language matrices induced by stenosis in the left and right ICA, 2-sample t tests were used to compare the FC among the aICSL, aICSR, and HC groups (P < .05, with false discovery rate [FDR] correction for multiple comparisons). The statistical power was estimated for the clinical features, altered FCs, and association between the clinical features and FCs found to significantly differ after aICS.
Partial correlation analysis (P < .05) was applied to evaluate the associations between immediate and delayed recall of verbal memory and altered FC in the anterior and posterior circulation territories induced by aICSL and aICSR. To regress out the effects of age and sex, these 2 characteristics were assigned as confounding factors during the test for partial correlation between verbal memory and altered FC.
RESULTS
Demographic Characteristics and Neuropsychological Data
Age, sex, degree of stenosis, and handedness did not significantly differ among the 3 groups. The aICSL group showed significantly reduced MMSE scores compared with the HCs (P = .002 with a statistical power of 0.94) and aICSR (P = .01 with a statistical power of 0.76) groups. The aICSL group showed a significantly poor level of reverse DST scores compared with the HCs group (P = .01 with a statistical power of 0.77). In the comparison of verbal memory scores, the aICSL group presented significant deficits in immediate recall of verbal memory compared with the HC group. In addition, both the aICSL and aICSR groups showed significantly lower scores on the verbal memory delayed recall test than the HC group. No significant difference in verbal memory function between the aICSL and aICSR groups was found (Table 1). In addition, we confirmed no significant difference in distribution of Fazekas scores among HC, aICSL, and aICSR groups (Table 2).
Demographic characteristics and neuropsychological scores of the study cohort
The distribution of the severity of white matter hyperintensities for aICSL and aICSR groups. The total Fazekas score is the summation of scores for periventricular white matter and deep white matter
Comparisons of FC among the aICSL, aICSR, and HC Groups
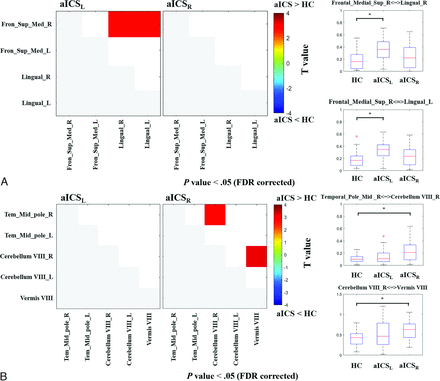
In the anterior circulation territory FC matrix, significantly higher FC between right superior medial frontal gyrus and left lingual gyrus (aICSL = 0.34 ± 0.15, HC = 0.20 ± 0.15, corrected P = .05 with a statistical power of 0.78), as well as right lingual gyrus (aICSL = 0.34 ± 0.18, HC = 0.19 ± 0.15, corrected P = .04 with a statistical power of 0.79), were observed in patients with aICSL than in HCs (Figure 3A). The comparison between the aICSR and HC groups showed no significant findings. In the posterior circulation territory FC matrix, significantly higher FC between right cerebellum VIII and the right middle temporal pole (aICSR = 0.24 ± 0.17, HC = 0.11 ± 0.06, corrected P = .05 with a statistical power of 0.90) as well as the right cerebellum VIII and vermis VIII (aICSR = 0.62 ± 0.24, HC = 0.41 ± 0.19, corrected P = .04 with a statistical power of 0.86), were observed in patients with aICSR than in HCs (Figure 3B). The comparison between the aICSL and HC groups showed no significant findings. In the comparison of FC between the aICSL and aICSR groups, no significant difference was observed in either the anterior or posterior circulation territory. Regarding the language network, no significant differences in FC were identified by comparing the aICSL or aICSR group with the HC group (Table 3).
The t maps from the comparisons of FC among the aICSL, aICSR, and HC groups. A, The matrices show the comparison between the aICS and HC groups in regions in the anterior territory. The boxplot presents the distribution of altered FC in the anterior circulation territory. B, The matrices show the comparison between the aICS and HC groups in regions in the posterior territory. The boxplot presents the distribution of altered FC in the posterior circulation territory. Red or blue nodes indicate that patients with stenosis in the right internal carotid artery show a significant increase or reduction in FC, respectively. Fron_Sup_Med: superior medial frontal gyrus, Tem_Mid_pole: middle temporal pole, R: right, and L: left. *P value < .05 (FDR corrected).
List of primary language regions with altered FC. The first 3 columns show the distribution of FC in the HC, aICSL, and aICSR groups. The fourth and fifth columns list FDR-corrected P values of comparison between the HC and aICSL groups and between the HC and aICSR groups, respectively
Correlation between FC and Delayed Recall of Verbal Memory
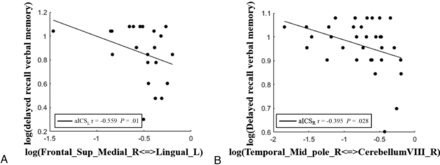
We observed negative correlations between FC and delayed recall of verbal memory, indicating that stronger FC is associated with a lower level of delayed recall of verbal memory. In the anterior circulation territory, the FC between the right superior medial frontal gyrus and left lingual gyrus showed a significant negative correlation with delayed recall of verbal memory in the aICSL group (r = −0.559, P = .01 with a statistical power of 0.99). In the posterior circulation territory, the FC between the right middle temporal pole and right cerebellum VIII showed a significant negative correlation with delayed recall of verbal memory in the aICSR group (r = −0.395, P = .03 with a statistical power of 0.99) (Fig 4).
Scatterplots showing correlations between the delayed recall of verbal memory and altered FC in the anterior and posterior vascular territories after aICSL and aICSR. The dots represent the strength of the altered FC and delayed recall of verbal memory for patients. The fitted lines are also displayed in the scatterplots. A, In the anterior circulation territory FC matrix, the FC between the right superior medial frontal gyrus and left lingual gyrus was significantly correlated with delayed recall of verbal memory after aICSL. B, In the posterior circulation territory FC matrix, the FC between the right middle temporal pole and cerebellum VIII was significantly correlated with delayed recall of verbal memory after aICSR.
DISCUSSION
In the current study, patients with either left or right aICS were observed to have lower delayed recall of verbal memory than the HC group. Although the laterality of aICS was not associated with the severity of decline in delayed recall of verbal memory, the stenosis side (in the left and right ICA) influences different FC changes in the anterior and posterior circulation territories compared with HCs. Correlation analyses indicated different functional compensations for decline in delayed recall of verbal memory after left or right unilateral aICS.
MMSE assesses comprehensive cognitive functions, including orientation, delayed recall, working memory, language, and visuoconstruction.30 DST is one of the most commonly used measures of attentional capacity, immediate verbal recall, and working memory, especially for memorization of number sequences.31 In this study, the aICSL group showed significantly reduced MMSE scores compared with HC and aICSR groups. The aICSL group also showed significantly poor levels of reverse DST compared with HC group. In general, multiple cognitive functions exhibited lateralization (left-hemisphere dominance), including language, memory, and logic processing.32 Accordingly, reduced perfusion from the left internal carotid artery may cause a significant influence on the cognitive functions assessed by the MMSE tests and reverse DST. However, the MMSE and reverse DST scores in the aICSL group showed no significant correlations with FC between selected brain regions.
The white matter hyperintensities in T2 FLAIR images indicated an increased risk of cerebrovascular events, stroke, and dementia and can be further employed to predict cognitive impairment.33 In this study, we found no significant difference in the distribution of Fazekas scores among HC, aICSL, and aICSR groups. Accordingly, the confounding effect of white matter hyperintensities on the comparisons among 3 groups is controlled in this study.
Recent evidence has shown that cerebrovascular disease is a major contributor to later-life dementia, accounting for up to 20% of cases of dementia.34 Therefore, research of vascular contributions to cognitive impairment and dementia (VCID) is growing. The proposed underlying mechanisms of VCID include cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling.35 Normal brain function depends on adequate blood flow supplied by cervico-cerebral blood vessels via competent neurovascular coupling.36 Severe stenosis of carotid arteries leads to impairment of cerebral perfusion. Cerebral autoregulation triggered by decreased cerebral blood perfusion modulates the distribution of blood flow.37,38 The altered perfusion from the ipsilateral ICA and contralateral vertebral artery compensates for cerebral perfusion in the affected regions to maintain cognitive function. A cohort study enrolling 19 patients with asymptomatic carotid stenosis and 24 heathy controls showed aICS-induced alteration of regional activation in compensatory regions, including the superior medial frontal gyrus, middle temporal pole, lingual gyrus, and cerebellum.39 The reported FC among compensatory regions in that study is considered to be related to the modulations in language, attention, visual, and memory networks.40 The superior medial frontal gyrus is involved in attention,17 while the lingual gyrus handles the processing of visual stimuli, especially letters.41 The middle temporal pole and cerebellum VIII participate in memory encoding.42 The current study showed that patients with aICS with enhanced FC among compensatory regions exhibit poor delayed recall of verbal memory, which may indicate that altered FC leads to inefficient delayed recall of verbal memory function. However, the negative correlation suggests that enhanced FC among compensatory regions indicates the severity of delayed recall of verbal memory decline following left or right aICS.
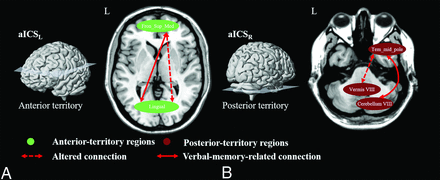
The effects of the laterality of aICS on structural and functional connections were less explored.12,39,43⇓⇓-46 We observed the aICSL and aICSR groups showed different patterns of association between verbal memory decline and altered FC in the anterior and posterior circulation territories, respectively (Fig 5). In this study, we suggest that the side of aICS may involve different compensatory mechanisms associated with the decline in delayed recall of verbal memory. In the patients with aICSL, we found that the compensation was presented by significantly increased FC related to the right superior medial frontal gyrus in the anterior circulation territory (Figure 3A). However, in patients with aICSR, we found different compensation presented by significantly increased FC related to the right cerebellum VIII in the posterior circulation territory (Figure 3B). We speculated that the differences in compensatory mechanisms between the aICSL and aICSR groups may be related the lateralization of verbal memory (ie, left-hemisphere dominance).32 While the cerebral perfusion in the left hemisphere (dominant regions of verbal memory) was reduced in the aICSL group, the primary auxiliary FC related to the contralateral superior medial frontal gyrus was increased as a response (Fig 5). He et al13 also observed the cross-hemispheric FC in the patients with unilateral left aICS. Contrarily, while the perfusion in the right hemisphere (auxiliary regions of verbal memory) was reduced in the aICSR group, the secondary auxiliary FC related to the ipsilateral cerebellum VIII was increased instead (Fig 5). Carlson et al47 reported that patients with severe perfusion reduction in the right hemisphere, such as caused by ischemic stroke, showed significant FC differences in the ipsilateral posterior circulation territory.
Diagram summarizing the underlying compensatory mechanism (neuroplasticity) after aICSL and aICSR. A, Anterior circulation territory compensation for verbal memory recall after aICSL. B, Patients with aICSR showed posterior circulation territory compensation for recall verbal memory. The green nodes indicate brain regions in the anterior circulation territory; the wine-colored nodes represent brain regions in the posterior circulation territory. The dashed double arrows indicate altered FC after aICS. The altered connections related to verbal memory recall are presented with double arrows.
A decline in verbal memory is one of the common complaints of patients with aICS. A previous review reported no evidence that the degree of stenosis was predictive of verbal memory decline.5 To evaluate the severity of verbal memory decline in the present study, patients with aICS completed a well-developed and well-validated Chinese version of the Verbal Learning Test.48 However, the period of rs-fMRI acquisition was approximately 20 minutes, which is shorter than the duration of a Verbal Learning Test. This study employed fMRI-based FC analysis to extract quantitative image biomarkers for evaluating functional remodeling following a disease.
This study employed the pretreatment fMRI data to separately explore the association between the altered FC and decline in delayed recall of verbal memory in the aICSL and aICSR groups. We aimed to unravel the influence of the aICS side on the verbal memory decline and investigate corresponding compensatory mechanisms. However, comparing FC changes between pretreatment and posttreatment fMRI for the aICSL and aICSR groups may provide new insights into the therapeutic effect of revascularization. Most previous studies performed the investigation by pooling the aICSL and aICSR groups,49,50 which may cause a mixing effect to prevent the identification of significant FC alterations. Previous literature has reported the inconsistent recovery of FC and verbal memory after the revascularization. Wang et al49 have reported the significant decreased activation in cerebellar regions in a group of 5 patients with aICSL and 11 with aICSR after the treatment. Quandt et al50 have suggested a more significant increase in FC within the contralateral hemisphere in a group of 8 patients with aICSL and 4 with aICSR after the treatment. These inconsistent findings may indicate the influences of aICS side on recovery of FC after the revascularization. Accordingly, the therapeutic effect and recovery mechanism associated with the side of aICS after the revascularization are worthy of further investigation.
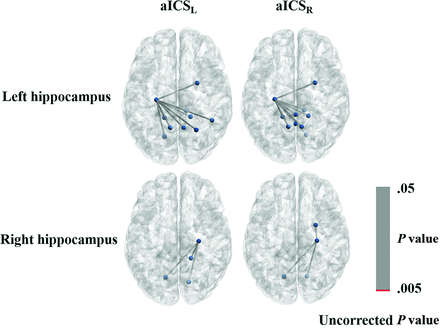
Several limitations of the current study are described as follows: First, no hippocampus-related imaging biomarkers for decline in delayed recall of verbal memory were observed (Fig 6). The hippocampal region is mainly perfused by branches of the posterior cerebral artery, which was not significantly affected after aICS.51,52 Our study indicated that functional compensation for decline in delayed recall of verbal memory after aICS may be associated with other brain areas, including frontal, temporal, and cerebellar regions. Second, although patients with insufficient blood perfusion in the left hemisphere may exhibit severe decline in delayed recall of verbal memory,53 the small sample size of this study may have impeded detection of significant differences in delayed recall of verbal memory decline between the aICSL and aICSR groups. We matched the variables, including age, sex, degree of stenosis, and handedness among the 3 groups. The statistical power in FC analysis achieved statistical power between 0.78 and 0.90, suggesting that our results are reliable even with a small sample size. However, further study with a larger sample size is required to consolidate the identified association between FC alteration and verbal memory decline.
Graph displaying the FC of the left or right hippocampus gyrus with uncorrected P values less than .05. The connections with P values less than .005 are shown in red; those connections with P values between .005 and .05 are shown in gray.
CONCLUSIONS
This study suggested that the side of aICS may influence the FC alterations in both the anterior and posterior circulation territories. Stenosis in the left or right ICA was associated with different compensatory mechanisms for the decline in delayed recall of verbal memory.
Footnotes
Jyun-Ru Chen and Chun-Jen Lin contributed equally to this article.
This work was funded by the National Science and Technology Council (NSTC 112-2314-B-A49-060, 112-2314-B-075-037), Veterans General Hospitals and University System of Taiwan Joint Research Program (VGHUST113-G1-2-3), and the Taipei Veterans General Hospital (V112B-010). The funding bodies played no part in the study design, data collection, analysis, interpretation, or manuscript preparation.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received December 6, 2023.
- Accepted after revision February 12, 2024.
- © 2024 by American Journal of Neuroradiology