Abstract
BACKGROUND: Hereditary hemorrhagic telangiectasia is an autosomal dominant vascular dysplasia characterized by mucocutaneous telangiectasias, recurrent epistaxis, and organ vascular malformations including in the brain, which occur in about 10% of patients. These brain vascular malformations include high-flow AVMs and AVFs as well as low-flow capillary malformations. High-flow lesions can rupture, causing neurologic morbidity and mortality.
STATE OF PRACTICE: International guidelines for the diagnosis and management of hereditary hemorrhagic telangiectasia recommend screening children for brain vascular malformations with contrast enhanced MR imaging at hereditary hemorrhagic telangiectasia diagnosis. Screening has not been uniformly adopted by some practitioners who contend that screening is not justified. Arguments against screening include application of short-term data from the adult A Randomized Trial of Unruptured Brain Arteriovenous Malformations (ARUBA) trial of unruptured sporadic brain AVMs to children with hereditary hemorrhagic telangiectasia as well as concerns about administration of sedation or IV contrast and causing patients or families increased anxiety.
ANALYSIS: In this article, a multidisciplinary group of experts on hereditary hemorrhagic telangiectasia reviewed data that support screening guidelines and counter arguments against screening. Children with hereditary hemorrhagic telangiectasia have a preponderance of high-flow lesions including AVFs, which have the highest rupture risk. The rupture risk among children is estimated at about 0.7% per lesion per year and is additive across lesions and during a lifetime. ARUBA, an adult clinical trial of expectant medical management versus treatment of unruptured brain AVMs, favored medical management at 5 years but is not applicable to pediatric patients with hereditary hemorrhagic telangiectasia given the life expectancy of a child. Additionally, interventional, radiosurgical, and surgical techniques have improved with time. Experienced neurovascular experts can prospectively determine the best treatment for each child on the basis of local resources. The “watch and wait” approach to imaging means that children with brain vascular malformations will not be identified until a potentially life-threatening and deficit-producing intracerebral hemorrhage occurs. This expert group does not deem this to be an acceptable trade-off.
ABBREVIATIONS:
- HHT
- hereditary hemorrhagic telangiectasia
- VM
- vascular malformation
In this report, a multidisciplinary group of hereditary hemorrhagic telangiectasia (HHT) experts and patient advocates from North America discuss the rationale for the international guideline adopted in 2019 and disseminated in 2020 that recommends MR imaging screening of children with HHT for brain vascular malformations (VMs).1 Brain VMs among children with HHT include AVMs and AVFs with arteriovenous shunting and the potential for rupture as well as nonshunting capillary malformations that are currently thought to have a more benign clinical course. Early screening allows prospective decision-making regarding treatment of lesions deemed at high risk for future bleeding. This practice contrasts with a “wait-until-symptoms” neuroimaging approach advocated by other groups.2 Since disabling or fatal hemorrhage can be the first symptom of a brain VM, we do not consider the “wait-until-symptoms” approach to be safe. We provide a medical summary of brain VMs in children with HHT and a list of pros and cons for screening for brain VMs in Tables 1 and 2, respectively.
Brain vascular malformations in children with HHT
Summary of pros and cons of brain VM screening for children with HHT
Background
HHT is an autosomal dominant genetic disease characterized by recurrent epistaxis, mucocutaneous telangiectasias, and organ VMs, including in the brain and lungs. This condition affects approximately 1 in 5,000–10,000 people worldwide.3,4 Eighty-five percent of those who meet clinical criteria have 1 of 3 genetic variants (ENG, ACVRL1, or SMAD4).5 At least 10% of all individuals with HHT have brain VMs.6 While the prevalence of brain VMs may be as high as 20% among those with ENG variants,7 no other genotype-phenotype correlations exist that predict which individuals are likely to have brain VMs or specific brain VM characteristics.8 Three different brain VMs have been described in those with HHT: single-hole pial high-flow brain AVFs, classic nidus-type brain AVMs, and nonshunting capillary malformations.9,10 These different types of brain VMs appear to have different natural histories, which may explain why the natural history of all subtypes of brain VMs in HHT, when aggregated, appears to be more benign compared with patients who have sporadic brain AVMs.11 Different types of brain VMs appear to have different age distributions, with a preponderance of high-flow AVFs in young children.9
Brain VMs can cause headaches and seizures, but vascular rupture with intracerebral hemorrhage is the principal cause of morbidity and mortality. Among those with HHT and brain AVMs, the risk of hemorrhage is about 0.4%–2% per lesion per year.11,12 The hemorrhage risk is even higher—at least 6%–10% per year—if there has been prior hemorrhage.12 Up to 39% of those with HHT and brain AVMs have multiple lesions, likely elevating the risk of hemorrhage because risk may be additive across all of the lesions.13 In 1 study of 221 children with HHT, almost 29% had brain VMs, of which about 36% were AVMs or AVFs. The remainder of brain VMs were low-flow lesions such as capillary malformations, cavernous malformations, and developmental venous anomalies.14 The annual hemorrhage risk in this pediatric cohort was 0.7% per year,14 approximating estimates in adults.
Current International Guidelines
Given the risk of hemorrhage and complications related to brain VMs, experts in HHT care published international guidelines for the diagnosis and management of HHT and recommended screening adults and children with HHT for brain VMs.1 For children, these guidelines specifically suggest screening asymptomatic children with HHT or those at risk for HHT at the time of presentation or upon diagnosis.1 Pediatric hemorrhagic stroke literature demonstrates a high mortality rate (estimates of 4%–54%) and substantial morbidity including motor, language, and cognitive impairments as well as chronic sequelae such as headache and epilepsy.15 Preventing intracerebral hemorrhage is therefore of paramount importance for the long-term health of children with HHT. The goal of brain VM screening is to identify brain VMs at risk of rupture so that these can be treated before a child experiences a life-threatening hemorrhage or permanent brain injury and neurologic sequelae. Despite published HHT guidelines and recommendations, a survey of 28 North American HHT Centers of Excellence demonstrated that many, but not all, centers screen children for brain VMs.16,17 Moreover, only about 80% of centers that screen include recommended contrast-enhanced MR imaging, the most sensitive sequence for detecting the sometimes small VMs in HHT.16,17 In this review, we address concerns that have been raised about brain VM screening in pediatric patients with HHT and provide evidence in support of brain VM screening in HHT and for its safety.
Arguments Against Screening for Brain VMs
There are several reasons that physicians and parents may be hesitant to perform brain VM screening. First, a perception exists that children with HHT only rarely experience complications from brain VMs or that children with brain VMs will show signs or symptoms of the VM before rupture and hemorrhage. However, while brain VMs are sometimes symptomatic before rupture, this may only be recognized retrospectively because headaches and focal seizures can be difficult to recognize in small children. Second, some may believe that the consequences of brain VM rupture are not as serious in the pediatric age group as in adults because children can recover due to “neuroplasticity.” However, there are well-described long-term sequelae of hemorrhagic stroke in childhood.15 Third, some inappropriately cite A Randomized Trial of Unruptured Brain Arteriovenous Malformations (ARUBA) to justify not screening for AVMs.18 This trial did not include children and followed subjects for a relatively short period of 5 years initially.18,19 Newer data20 suggest that extrapolation to children, who have a much longer life expectancy than adults, is not only inappropriate but truly incorrect. Finally, some are concerned about potential complications of sedation on the developing brain or of the sedation itself (eg, aspiration, cardiac arrest), complications of MR imaging contrast, the need for reimaging smaller, nonsurgical lesions that may require follow-up, or about the procedural safety of conventional angiography.
Arguments in Favor of Screening for Brain VMs
Risks of Brain Hemorrhage in Children with HHT.
The risk of intracerebral hemorrhage due to AVMs or AVFs in children with HHT is marked and can occur early in life, as demonstrated in Fig 1. AVFs, which are enriched in the pediatric HHT population, carry the highest risk of rupture among brain VMs. While brain AVMs in HHT are often thought to be less dangerous than non-HHT brain AVMs, this perception is likely due to the fact that most published estimates of hemorrhage risk among patients with HHT pool all brain VM types, including low-flow capillary malformations which have a very low hemorrhage risk, thereby decreasing this pooled risk estimate.11 In fact, the risk of hemorrhage among children with HHT is much higher than that in the general pediatric population. A recent study that used 2 large databases, including a multistate Medicaid database, demonstrated that among 588 children with HHT younger than 16 years of age, there were 189.7 cases of hemorrhagic stroke or SAH per 100,000 child years, with a relative risk of hemorrhagic stroke or SAH of more than 60 compared with matched children without HHT.21 The report by Azma et al14 demonstrated a risk of hemorrhage of about 0.7% per year among children with HHT, and 7 of the 211 children in the cohort (3.2%) had hemorrhage. These hemorrhagic strokes occurred at a median of just 2 years of age (interquartile range, 1.3–6.7 years),14 which fits well into the observed age-dependency of high-flow VMs like AVFs, which are typically more dangerous and present in the young age group.9
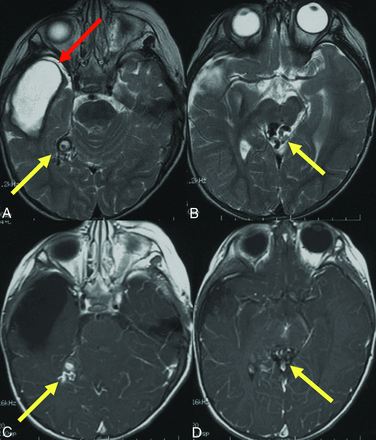
Prior brain hemorrhage in an infant with HHT. T2-weighted MR imaging (A) demonstrates a hemosiderin-lined cavity (red arrow) in the right temporal lobe of a 15-month-old girl with HHT. Additional AVFs (yellow arrows) are evident on T2 (A and B) and contrast-enhanced T1-weighted images (C and D).
Several other series suggest that a high proportion of children with brain AVMs have intracerebral hemorrhage. In 1 report of 115 children with HHT, 11 (9.6%) had brain AVMs, and 4 of these 11 children (36.3%) had intracerebral hemorrhage as their initial clinical presentation of HHT.22 In another cohort of 52 children with HHT who had brain imaging, 14 had brain VMs (26.9%), including 7 with brain AVMs and 2 others with high-flow vein of Galen malformations. Three of the 7 children with brain AVMs (50%) had intracerebral hemorrhages, also at young ages, between 4.3 and 7.7 years; one of these 3 children died.23 While survival methods were not used in the studies by Saleh et al22 and Beslow et al,23 the young ages of the patients with few person years to contribute to the study time indicate that the risk of hemorrhage among pediatric patients with HHT and brain VMs may actually be higher than among adults, potentially reflecting survival bias in adulthood. This premise is supported by data from the Brain Vascular Malformation Consortium HHT database.24 When comparing 114 children with HHT and brain VMs with 253 adults with HHT and brain VMs, children were more likely to have headaches (29.8% versus 20.9%), seizures (18.4% versus 7.5%), and intracerebral hemorrhage (23.7% versus 9.9%) and were less likely to have been diagnosed by screening (56.1% versus 66.4%).24 As noted, one reason for the high proportion of children with HHT who present with intracerebral hemorrhage may be the preponderance of high-flow AVFs in the pediatric age group compared with adults with HHT.9
In addition to evidence that brain VMs in children with HHT may have different clinical features than those found in adults with HHT, the underdiagnosis of HHT likely impacts the understanding of hemorrhage risk. Children with HHT who present with hemorrhage but have not yet been diagnosed with HHT may not be included in HHT-related hemorrhage risk estimates. In a cohort of 89 pediatric patients without a personal or family history of HHT who presented to a large tertiary care center with brain AVMs, AVFs, or vein of Galen malformations and who underwent evaluation for HHT as part of an institutional protocol, 13 (14.6%) had definite HHT, of whom 9 had causative HHT genetic variants.25 Of note, 6 of 13 (46%) with definite HHT and brain VMs presented with VM rupture (at an age range of <1 month to 14 years).25 (Personal communication with Engel and Hammill, October 2023) These data underscore that brain VMs can be a presenting feature of HHT among children. These data also indicate that HHT is likely underdiagnosed among pediatric patients with symptomatic high-flow VMs because genetic testing for lesions thought to be sporadic is not routinely performed at many institutions.
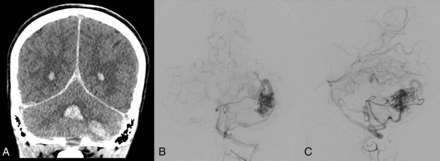
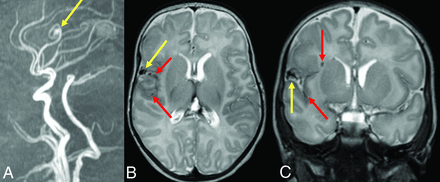
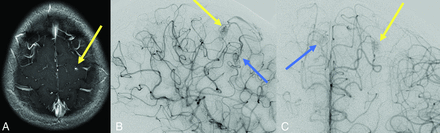
The findings of Engel et al discussed above can also be interpreted to support screening for brain VMs in children with HHT. Even if all 13 children with high-flow brain VMs and HHT had been diagnosed with HHT before brain VM clinical presentation, almost one-half would have presented with hemorrhagic stroke in the absence of screening,25 a number that the authors deem unacceptably high. Figure 2 shows images from a child with HHT who presented with a life-threatening cerebellar hemorrhage due to a brain AVM. The AVM could have been successfully treated before the hemorrhage occurred if it had been detected on screening. Figure 3 shows images from a 2-month-old infant whose pial AVF was identified on screening and who underwent successful embolization at 6 months of age when surveillance imaging demonstrated progression. The embolization was without complications and resulted in obliteration of the AVF. Findings of repeat imaging performed 7.5 years after treatment were stable without AVF recurrence. The child is neurologically and cognitively healthy at 9 years of age. Figure 4 shows MR imaging and subsequent DSA from a girl with ENG-related HHT. The DSA demonstrated the subtle finding on MR imaging in greater detail and identified a second small AVM that was not visible on MR imaging.
A 13-year-old boy with HHT who presented with severe headache and altered mental status. A, Coronal noncontrast head CT shows an acute left cerebellar hemisphere hemorrhage and intraventricular hemorrhage in the bilateral lateral ventricles and fourth ventricle. Anterior-posterior (B) and lateral (C) views on digital DSA depict a left cerebellar AVM with arterial supply from the left anterior and posterior inferior cerebellar arteries, left superior cerebellar artery, and deep venous drainage through the transverse sinus.
An infant diagnosed with HHT due to a familial ENG variant who had screening MR imaging of the brain and MRA of the head at 2 months of life had a pial AVF with hemosiderin deposition. Sagittal reformat (A) TOF-MRA demonstrates the right fontal AVF (yellow arrow). Axial (B) and coronal (C) T2-weighted MR images confirm the location of the AVF (yellow arrows) as well as adjacent hemosiderin deposition (red arrows) suggestive of prior hemorrhage.
A girl diagnosed with HHT due to a familial ENG variant had screening MR imaging that revealed a punctate area of enhancement in the left posterior frontal lobe surrounded by a subtle halo of enhancement (yellow arrow) on a high-resolution 3D T1 postgadolinium image (A). This finding corresponds to a subcentimeter AVM nidus (yellow arrow) on lateral (B) and anterior-posterior (C) DSA. An additional subcentimeter AVM nidus (blue arrow) supplied by the contralateral anterior cerebral artery is also identified on DSA.
Inappropriate Application of ARUBA Trial Data.
The ARUBA trial evaluated whether surgery versus medical care had a lower risk of stroke and death for adults with sporadic (non-HHT) unruptured brain AVMs and demonstrated that medical management alone was superior at a mean of 33.3 months and at a mean final follow-up of 50.4 months.18,19 Relying heavily on the ARUBA data, the European Reference Network for Rare Vascular Diseases (VASCERN) group does not recommend widespread brain VM screening for asymptomatic adults or children with HHT, citing limited data for treatment of unruptured AVMs.2 Data from the ARUBA study are not directly relevant to children with HHT; ARUBA did not include children, so its results should not be extrapolated to the pediatric population or to the pediatric population with HHT.18 The ARUBA trial also had a short time horizon, with a mean final follow-up of <5 years.18,19 Emerging evidence demonstrates that across a longer time period, survival curves can favor intervention for unruptured brain AVMs.20 For children, an age group with a life expectancy of several decades, intervention is therefore expected to be beneficial over a lifetime. Other drawbacks of ARUBA include its small sample size, which was merely one-half the enrollment goal, an ambiguous primary end point, and heterogeneous interventions, whereas now we know the best treatment is often a combination of surgery, embolization, and radiation and is dependent on individual patient and AVM features. Furthermore, a comparison of 32 children and 192 adults who underwent microsurgical brain AVM resection demonstrated better outcomes among children though clinical and anatomic features and treatment techniques were not different.26 This study again underscores that the results of ARUBA should not be applied to children.
The VASCERN group, which recommends against widespread testing but also recommends sharing current evidence with families,2 does not appear to emphasize sharing the many limitations of the ARUBA trial or evidence from pediatric cohorts. Additionally, radiographic evidence of asymptomatic, prior hemorrhage in adults with sporadic brain AVMs (ie, hemosiderin present in or around the AVM) portends an aggressive course, and patients with brain AVMs without frank rupture but who have silent hemorrhages may benefit from surgical treatment despite the data from ARUBA.27 Currently, the only method to detect silent hemorrhages and thus to identify patients who may be at higher risk for rupture is to image with iron-sensitive MR images. This information further suggests that imaging children with HHT is warranted not only to identify brain VMs but also to evaluate signs of silent hemorrhage. Notably, a recent study of 154,297 brain AVM admissions from the National Inpatient Sample demonstrated that since the publication of the ARUBA results in 2014, there has been a decrease in treatment of unruptured brain AVMs and a concomitant increase in hemorrhage rates,28 a concerning finding that should cause those who are hesitant about screening children with HHT to reconsider their position.
Importantly, treatments for brain AVMs and other brain VMs continue to expand and improve. As treatment options develop, including targeted medications, it will become more important to identify brain VMs. Experienced neurosurgeons and neurointerventionalists are able to assess the risks of procedures and individualize treatment plans on the basis of AVM location, AVM features, and patient age, including for patients with HHT-related brain AVMs.29⇓⇓-32 Data from the Brain Vascular Malformation Consortium demonstrate that children with HHT are more likely to have surgical treatment alone or embolization alone and are less likely to have radiosurgery alone than adults with HHT.24 These age-based treatment differences may reflect the higher risk of symptomatic clinical presentations of brain VMs in children, including hemorrhage, differences in brain VM type and structure between children and adults, and finally the impracticality of radiosurgery in very young children whose skulls may not fit in stereotactic frames designed primarily for adults. Detailed natural history data and imaging repositories are needed to understand the features of HHT-related brain VMs better and track outcomes accurately after brain VM treatments. While screening may identify low-flow lesions that have a low risk of rupture or small AVMs that may not yet warrant treatment, the knowledge that a lesion is present allows serial monitoring for growth or signs of hemorrhage, so that treatment options can be offered and discussed when and if appropriate.
Patient and Family Anxiety.
Another concern that some cite as a rationale for not screening children is the possibility of causing families anxiety.2 While there are studies that demonstrate psychological distress and decreased quality of life among patients with HHT,33⇓-35 there are no pediatric-specific quality of life studies or research that have examined the impact of screening procedures themselves. We acknowledge that identifying a smaller malformation that does not require treatment or a larger one that is not amenable to treatment (eg, a deep brain AVM with a high Spetzler-Martin score) may cause anxiety for some children and their families. It is not, however, the physician’s role to avoid sharing information that may make someone anxious. Not sharing information is paternalistic and does not respect patient and family autonomy. It is, instead, the physician’s duty to educate parents and patients with data so that informed shared decision-making can take place.
Risks of Sedation and Imaging Procedures.
Any test or procedure has risks. However, methods can be used to minimize the risks of sedation and anesthesia, MR imaging contrast, and cerebral angiography. Many pediatric hospitals have expert presedation and preanesthesia teams that carefully review each child’s history so that sedation or anesthesia complications can be minimized. For the HHT population in particular, children with frequent epistaxis or known pulmonary AVMs are often triaged to general anesthesia rather than to a sedation team. Charts are reviewed in detail, and careful histories are taken to ensure that no viral symptoms or other symptoms of acute illness are present that may prompt rescheduling. Excellent reviews exist that discuss sedation and various anesthetics for pediatric MR imaging.36 In 1 study of nearly 50,000 non-operating room propofol sedations in children, there were only 2 cardiorespiratory arrests, 4 cases of aspiration, and no deaths.37 In another study of 276,832 sedations, the risk of laryngospasm was low at 3.3 per 1,000 sedations.38 In all, the risk of respiratory distress and other acute severe complications from sedation is very low, and these are typically non-life-threatening and merely require additional monitoring or observation.
Regarding MR imaging gadolinium contrast, the risk is low. However, many high-flow AVMs and AVFs can be identified on T2-weighted imaging without contrast, though noncontrast imaging might miss low-flow capillary malformations or smaller AVMs that are visible on contrast-enhanced MR imaging.17 Thus, there remains variation among centers with regard to use of contrast despite the recommendation of the guidelines that MR imaging screening be performed with and without contrast. A sample screening MR imaging–based protocol is presented in Table 3.
Sample MR imaging–based protocol for brain VM screening in children with HHT (see also Vella et al17)
Complications of conventional angiography can include trauma to the femoral artery, including hematoma or, pseudoaneurysm, and dissection or rupture of neck vasculature. These complications are rare in the pediatric population, especially when the procedures are performed by angiographers experienced with pediatric patients. In 1 study of 390 children who underwent 587 consecutive cerebral angiography procedures between 2002 and 2020, complications occurred in 6.5%.39 Major complications occurred in 0.5% of procedures, and permanent deficits occurred in 0.2% of cases.39 Catheters appropriate for smaller-sized pediatric patients are available in pediatric centers and are stocked in procedure rooms. Established postprocedural protocols exist to minimize complications, such as the use of pressure bandages and lying flat for 6 hours to minimize hematoma or local pseudoaneurysm formation. In experienced pediatric centers, children with HHT can undergo screening for brain VMs safely, with minimal sedation and procedural risks.
CONCLUSIONS
The 2020 international guidelines for HHT recommend screening children with HHT for brain VMs at HHT diagnosis or presentation.1 Children with HHT have a 60-fold higher risk of hemorrhagic stroke and SAH compared with children without HHT,21 a startling finding that emphasizes the need to detect brain VMs, assess their risk, and determine treatment options versus serial monitoring. The practice of waiting for children to present with a stroke before obtaining imaging is supported neither by evidence nor by the recommendations from the guidelines committee1 and has allowed an unnecessarily high risk of serious morbidity and mortality to persist.
After screening, any child identified to have a brain VM should be discussed by a multidisciplinary team, including a pediatric neurosurgeon or vascular neurosurgeon, neurointerventionalist, and pediatric neurologist to facilitate an individualized approach that minimizes complications. A clear discussion of the HHT guidelines and the available evidence should be presented to each family of a child with HHT so that shared decision-making can occur.
Footnotes
L.A.B. is funded by a Children’s Hospital of Philadelphia Department of Pediatrics Chair’s Initiative.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received November 21, 2023.
- Accepted after revision January 8, 2024.
- © 2024 by American Journal of Neuroradiology