Abstract
BACKGROUND AND PURPOSE: Successful post-flow-diverter endoluminal reconstruction is widely believed to require endothelial overgrowth of the aneurysmal inflow zone. However, endothelialization/neointimal overgrowth is a complex process, over which we currently have very limited influence. Less emphasized is vascular remodeling of the target arterial segment, the dynamic response of the vessel to flow-diverter implantation. This process is distinct from flow modifications in covered branches. It appears that basic angiographic methods allow simple and useful observations. The purpose of this article was to quantitatively evaluate observable postimplantation changes in target vessels following deployment of Pipeline endoluminal constructs.
MATERIALS AND METHODS: One hundred consecutive adults with unruptured, previously untreated, nondissecting aneurysms treated with the Pipeline Embolization Device with Shield Technology and the availability of follow-up conventional angiography were studied with 2D DSA imaging. Target vessel size; Pipeline Embolization Device diameter; endothelial thickness; and various demographic, antiplatelet, and device-related parameters were recorded and analyzed.
RESULTS: The thickness of neointimal overgrowth (mean, 0.3 [SD, 0.1] mm; range, 0.1–0.7 mm) is inversely correlated with age and is independent of vessel size, smoking status, sex, and degree of platelet inhibition. The decrease in lumen diameter caused by neointimal overgrowth, however, appears counteracted by outward remodeling (dilation) of the target arterial segment. This leads to an increase in the diameter with a corresponding decrease in length (foreshortening) of the implanted Pipeline Embolization Device. This physiologic remodeling process affects optimally implanted devices and is not a consequence of stretching, device migration, vasospasm, and so forth. A direct, linear, statistically significant relationship exists between the degree of observed outward remodeling and the diameter of the implanted Pipeline Embolization Device relative to the target vessel. Overall, remodeled arterial diameters were reduced by 15% (SD, 10%) relative to baseline and followed a normal distribution. Clinically relevant stenosis was not observed.
CONCLUSIONS: Vessel healing involves both outward remodeling and neointimal overgrowth. Judicial oversizing could be useful in specific settings to counter the reduction in lumen diameter due to postimplant neointimal overgrowth; however, this overszing needs to be balanced against the decrease in metal coverage accompanying the use of oversized devices. Similar analysis for other devices is essential.
ABBREVIATIONS:
- NIO
- neointimal overgrowth
- PED
- Pipeline Embolization Device
- PRU
- VerifyNow P2Y12 reactivity
- SW
- Shapiro-Wilk
Flow diversion has revolutionized the treatment of intracranial aneurysms.1,2 In large part, the success of devices such as the Pipeline Embolization Device (PED; Medtronic) stems from the endoluminal reconstruction of the aneurysm neck and affected vascular segment as opposed to strategies solely directed at the aneurysm sac. Multiple publications have addressed aspects of flow-diverter endothelialization or neointimal overgrowth (NIO) (the lining may be composed of multiple tissue types) believed, under most circumstances, to be responsible for the ultimate cure of targeted aneurysms.3,4 The complex relationship between alterations in intra-aneurysmal flow and NIO demands further consideration.5,6 Although potential avenues are being explored,7 our current influence on the NIO rate, extent, and long-term durability in the flow-diversion setting is limited. Antiplatelet therapy and surface modification reduce the probability of thrombus-related incidents, but these features may have little to do with NIO.8 Endothelial or intimal hyperplasia, terms used to describe a state of excessive endothelialization for clinical or research purposes, lack clear biologic distinction from normal or desirable endothelialization. We have limited ability to curtail intimal hyperplasia pharmacologically, such as with drug-eluting balloons. However, even this technology is difficult to deploy in most intracranial locations where flow diverters are used. Apart from building flow-diverter constructs of presumably optimal metal coverage and possibly surface modification, we have no way of promoting desirable NIO at this point.4,9⇓-11
There is no meaningful definition of endothelial hyperplasia with respect to flow-diversion therapy; the 50% threshold for recordable stenosis in most trials is an arbitrary number, intentionally set below clinical significance in most settings.12⇓-14 Most important, despite more than a decade of flow-diverter use, we lack objective benchmarks for normal or expected endothelial thickness/NIO, and their variability for any device presently on the market. While some instances of intimal hyperproliferation can be correlated with suboptimal deployment (malapposition and so forth), many occur without identified technical errors.15 Nearly all are asymptomatic, and a substantial portion improve spontaneously with conservative management.13,16
Another important element of the vascular response to flow-diverter implantation is remodeling, defined as an adaptation in target vessel diameter, compliance, and likely other properties following implantation of a foreign body. The clinical and academic focus regarding remodeling has been concentrated on the fate of jailed branches and their collateral circulation. Deliberate, sometimes stepwise coverage of branches associated with target aneurysms can be used to drive development of the circle of Willis and other collaterals, strategically resulting in aneurysm occlusion with preservation of cerebral perfusion.17⇓⇓⇓-21 However, this article addresses a different aspect of remodeling among the spectrum of posttreatment changes in the target vessel, which is under-reported in the flow-diverter literature.
Simple morphologic data like NIO thickness, pre- and posttreatment diameter of the target artery, and flow-diverter diameter and length are readily available from conventional angiography. Although optical coherence tomography offers superior measurement and tissue-differentiation capabilities, it is not clinically available.22 Also known are various antiplatelets, device sizes, and demographic data that could influence healing. The purpose of this study was to analyze this information, in hopes of establishing parameters for a normal and desired healing response and determine if any of these can influence change in a favorable direction.
MATERIALS AND METHODS
In an institutional review board–approved retrospective cohort study setting, we collected data on 100 consecutive fusiform and saccular brain aneurysms treated with single and multiple PEDs for which follow-up conventional angiography was available. Dissecting, previously ruptured, or treated aneurysms were excluded. All patients were premedicated with a dual antiplatelet regimen (aspirin, 81 mg daily, and clopidogrel, 75 mg daily). Preprocedural VerifyNow P2Y12 reactivity (PRU) was tested universally, and nonresponders were converted to ticagrelor (45–90 mg, twice daily, depending on sensitivity to the drug), with follow-up VerifyNow testing to ensure a response. Dual antiplatelet therapy was continued for at least 6 months posttreatment, followed by aspirin, 81 mg daily, or every other day monotherapy for varying periods depending on the clinical setting and preference of the treating physician.
Baseline demographics and derived values are shown in the Table. Specifically collected were discharge platelet reactivity values; device size and number; follow-up occlusion status; parent vessel diameter on initial 2D DSA and immediately preimplantation (to account for possible manipulation-related spasm), and immediately postimplantation; PED diameter immediately postimplantation, and PED and vessel diameter on follow-up 2D DSA obtained at least 5 months posttreatment. Five measurements of these parameters along the length of the treated segment were made for each metric and averaged, comparing averages pre- and posttreatment (Fig 1). Angiographic data were obtained on Artis Q and Icono biplane machines (Siemens). All length measurements were made on the Visage Client Pacs System (Version 7.1.18; PRO Medicus Limited). Measurements were correlated with those obtained directly from biplane units (both units undergo routine maintenance calibration) and with internal references (intermediate catheters) to ensure lack of systematic error. Two authors measured 10 cases together to establish a common measurement technique, with the remaining 90 cases measured by 1 of the 2 authors, with the second author reviewing results for internal consistency. Conebeam CT was not used due to its unavailability for most follow-up angiograms performed in an awake setting.
| Parameter | No. | SD |
|---|---|---|
| Age | 54 | 14 |
| Female sex | 81 | |
| Petrous | 1 | |
| Cavernous | 6 | |
| Paraophthalmic/paraclinoid | 66 | |
| PcomA | 7 | |
| Anterior choroidal | 3 | |
| MCA | 3 | |
| A1 | 1 | |
| AcomA | 7 | |
| Pericallosal | 2 | |
| PICA | 2 | |
| Basilar | 2 | |
| Fusiform aneurysms, No. | 3 | |
| Smokers | 27 | |
| Discharge PRU, average | 84 | 59 |
| Aneurysm size, average (mm) | 5 | 2 |
| No. of PEDs, average | 1.7 | 0.7 |
| Follow-up length, average (mo) | 9 | 3 |
| No. complete occlusions | 87 | |
| Neointimal thickness, overall (mm) | 0.3 | 0.1 |
| Neointimal thickness, 1 PED construct (n = 48) (mm) | 0.3 | 0.1 |
| Neointimal thickness, 2 PED constructs (n = 39) (mm) | 0.3 | 0.1 |
| Neointimal thickness, 3+ PED constructs (n = 13) (mm) | 0.3 | 0.1 |
| Increase in PED diameter post-Rx (mm) | 0.14 | 0.2 |
| Percentage change in target vessel diameter, pre-/post-Rx | −15 | 10 |
Note:—PcomA indicates posterior communicating artery; AcomA, anterior communicating artery; Rx, treatment.
Baseline demographic characteristics and treatment results
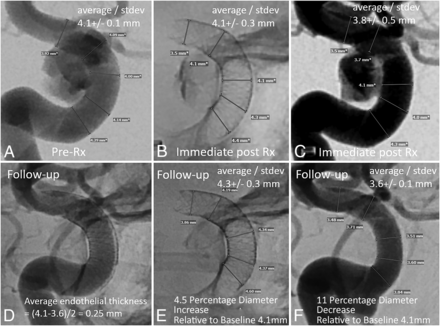
Example of target artery and PED diameter measurements before (A–C) and after (D–F) treatment. The immediate post-Rx DSA measurements (C) were not used for analysis. They are, however, indicative of the degree of procedure-related vasospasm. The PED is larger in diameter on follow-up (E) compared with immediately after implantation (B), despite a lack of vasospasm when C is compared with A. The expansion of PED in E compared with B is due to outward remodeling, which counteracts the endothelialization-driven decrease in follow-up vessel diameter (F). Rx indicates treatment.
Statistical Analysis
Demographic data were expressed as mean (SD) for continuous variables and percentage for categoric variables. Distributions of binned parameters were plotted as histograms, and relationships, as scatterplots. The more rigorous Shapiro-Wilk (SW) (compared with the less rigorous Kolomogorov-Smirnov) goodness-of-fit test was used to assess deviation from normal distribution.23 For the SW test, a P value < .05 rejects the hypothesis of normal distribution.
Pair-wise correlations were calculated as Pearson correlation coefficients using Excel (Microsoft); 95% confidence intervals were obtained using the Fisher transformation. Confidence intervals and SDs were reported, and a P value < .05 was considered statistically significant. Linear trendline and corresponding R2 values were obtained for the Online Supplemental Data, and ordinary least squares regressions were performed using the same software.
RESULTS
Background demographic, device, aneurysm, target vessel, and PED information as well as key results are shown in the Table.
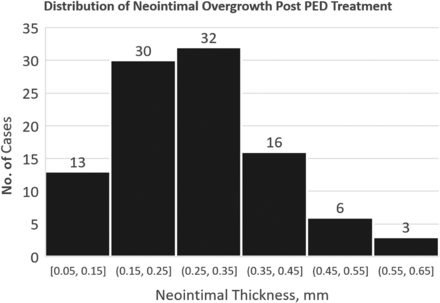
NIO thickness (0.3 [SD, 0.1] mm; 95% CI, 0.1–0.6 mm; range, 0.1–0.7 mm, Fig 2) is not distributed normally (SW test: W = 0.972, P = .03), ie, a skewed distribution with the tail to the right (thicker NIO lining).
Distribution of NIO thickness on follow-up imaging. There is no statistical indication that subjects with greater NIO thickness represent a different population; there is no second peak. It is not clear if substantially larger samples would change this assessment.
NIO is independent of vessel diameter, smoking status, discharge PRU value, number of implanted devices, or aneurysm occlusion status. The corresponding correlation coefficients and 95% CIs are vessel diameter (r = −0.02, P = .82; 95% CI, −0.1771−0.2156), number of implanted devices (r = −0.01, P = .89; 95% CI, r = −0.18−0.20), PRU at discharge (r = −0.01, P = .92; 95% CI, −0.18−0.22), occlusion status (r = −0.05, P = .63; 95% CI, −0.19−0.22), sex (r = 0.06, P = .59; 95% CI, 0.16−0.23), and smoking (r = −0.07, P = .48; 95% CI, −0.15−0.24). The only significant correlation was with patient age (r = −0.21, P = .04; 95% CI, −0.381 to −0.004), implying an overall negative association between age and NIO (Online Supplemental Data).
The change in PED diameter on follow-up angiography, compared with immediate postimplantation, is shown in the Online Supplemental Data. Most PEDs expand in diameter (0.14 [SD, 0.2] mm) on follow-up (Fig 1). This expansion is not fully explained by faulty implantation (stretching or vasospasm) because most delayed PED average diameter is greater than the average diameter of the parent vessel before implantation, proving that at least some component of outward remodeling (vessel dilation) occurs posttreatment (Online Supplemental Data). The distribution is normal by the SW test (W = 0.980, P = .12).
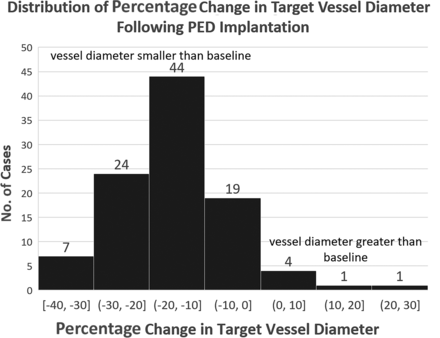
Figure 3 shows a 15% average reduction in healed vessel caliber posttreatment, with an SD of 10%, and normal distribution (SW test: W = 0.978, P = .09). No instance of >50% narrowing was observed in our sample.
Distribution of percentage change in target artery diameter compared with the pretreatment baseline. Most vessels are smaller in diameter, while a few are larger. The final diameter is governed by opposing factors of endothelialization and outward remodeling.
The ability of an artery to outwardly remodel is predicated on implantation of a device with the nominal diameter larger than that of the target artery, thus allowing subsequent expansion. To quantitatively evaluate this situation, we defined oversizing as the difference between the diameter of the target vessel and that of the implanted PED or the smallest-diameter PED for multidevice constructs.
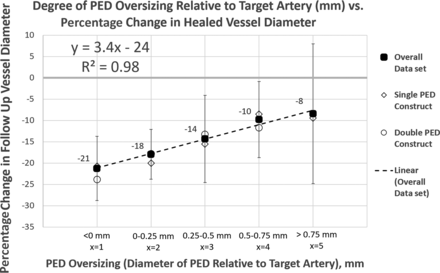
The relationship between oversizing and percentage change in artery diameter posttreatment is shown in Fig 4 for the overall data set and separately for single and double PED constructs, demonstrating nearly identical remodeling behavior. A similar relationship between percentage PED oversizing and percentage change in vessel diameter is shown in the Online Supplemental Data. There was not enough data for 3+ PED constructs for meaningful analysis.
PED oversizing relative to target artery versus percentage change in target artery diameter on follow-up DSA. There is an extremely robust (R2 = 0.98) linear relationship. Oversizing allows the vessel to expand (outward remodeling), thus mitigating an endothelialization-related decrease in vessel diameter. To what extent remodeling is influenced by device or construct radial force is unclear. The observation that single and double PED constructs behave similarly may suggest that radial force is not a major factor for PEDs in this range. However, possible differences may exist when baseline arterial diameter is considered by analyzing percentage PED oversize rather than absolute value (Online Supplemental Data). All findings are applicable to the PED only.
There is a strong, statistically significant linear relationship between PED oversizing and subsequent vessel diameter at follow-up angiography as shown by univariate ordinary least squares regression analysis. While the absolute luminal diameter of the posttreatment artery is most often reduced compared with the pretreatment diameter, greater oversizing is associated with a lesser degree of posttreatment vessel lumen reduction.
A 10% increase in PED diameter (oversizing) relative to vessel diameter is associated with a 3.1% relative increase in vessel size at follow-up (P < .01; 95% CI, 0.14–0.48) (Online Supplemental Data). In absolute lengths, oversizing the PED by 0.5 mm is associated with a 6% relative gain in vessel diameter (P < .01; 95% CI, 6.88–17.01), a potentially useful rule of thumb (Fig 4).
DISCUSSION
This study establishes a number of fundamental metrics and relationships pivotal to our understanding of healing after PED implantation. We quantitatively demonstrate that the treated arterial segment undergoes outward or positive remodeling (expansion, dilation) after PED treatment. This process, along with NIO, jointly determines the final vessel caliber. The concept of arterial remodeling is well-known in general vascular literature.24 However, most studies are focused on atheromatous disease. The presumably nonatheromatous nature of most vessels affected by intracranial aneurysms, especially the saccular type, represents a unique group in which remodeling can be studied apparently independent of atherosclerosis.
There is a strong correlation between the size of the device and the degree of remodeling. We may directly influence the degree of outward remodeling by the choice of PED diameter relative to the target arterial segment. In contrast, the degree of NIO currently seems independent of modifiable factors such as device number, vessel caliber, extent of platelet inhibition, or smoking habits. Interest in the development of biologically active surface modifications aimed at influencing endothelialization is currently high.
We observed a reduction in average neointimal thickness with increasing age, a phenomenon not previously reported to our knowledge for nonatheromatous intracranial implants, though this has been documented for intracranial atherostenotic disease.25
This observation suggests a possible muted (or less exuberant) biologic response with increasing age, highlighting the importance of gathering data for patients younger than 20 years of age (there were none in this sample).
The ability of the vessel to balance endothelialization with outward remodeling is remarkable and has significant practical implications. Opting for a device that is 0.5–0.75 mm larger than the target artery seems reasonable from a remodeling perspective but may be associated with lower metallic coverage of the aneurysm neck.
Prior research indicates that oversizing decreases metal coverage/pore density, potentially diminishing treatment efficacy while promoting branch vessel patency.9,10,26 This issue is particularly relevant for larger-diameter PEDs, which inherently have less metal coverage. Ensuring optimal neck coverage (with multiple devices if necessary) and maximizing treatment efficacy should take precedence over outward remodeling potential in most cases.9
In smaller vessels, the interplay between NIO and remodeling may be more important. Because NIO thickness does not seem to depend on target vessel size, it proportionately narrows smaller vessels to a greater degree. This narrowing can be mitigated by judicious oversizing, especially because small-diameter PEDs offer higher degrees of metal coverage even when oversized.
Oversizing can also negatively impact conformability, the ability of a braided device to adjust to changes in parent vessel diameter and curvature or across wide necks or fusiform aneurysms. Our recommended judicious oversizing by 0.5–0.75 mm considers the balance between the positive effects of oversizing and its impact on metal coverage and device conformability.4
The use of multiple PEDs does not adversely affect NIO thickness or the degree of vessel remodeling, with the extent of outward remodeling appearing similar in double and single PED constructs (Fig 4 and Online Supplemental Data).
In striving for an objective definition of endothelial (or neointimal) hyperplasia, the primary consideration is a clinical event. However, these are often multifactorial, while reporting may be incomplete. From a statistical perspective, given the normal distribution of the posttreatment diameter change, 95% of follow-up diameters should fall within −35% and 5% of baseline, and 99.7%, within −45% and 15%. Thus, 50% stenosis should be an extremely rare occurrence; none were observed in our sample, though literature reports vary widely.1,27,28 Using a 45% reduction in vessel diameter as the threshold of hyperplasia appears to be a statistically valid approach.
Limitations
The findings are strictly limited to the PED (with Shield Technology; https://shieldcctv.com/). Differences among devices in terms of material (Drawn Filled Tubes, nitinol), treatment efficacy, elasticity, and posttreatment healing are likely. It is essential to gather comparable data for other devices.
This study does not address posttreatment vessel factors other than diameter, such as vessel compliance, elasticity, and pulsatility. Despite a relatively strong correlation between device oversizing and healed vessel diameter and a statistically significant inverse correlation between endothelial thickness and age, the individual variations are large. Therefore, case-specific device-size selection should be made with the overall clinical scenario and treatment goals in mind. A larger number of subjects would be needed to perform multivariate regression analyses to further investigate factors associated with NIO thickness.
The study included only patients with follow-up angiography, potentially missing cases of asymptomatic stenosis or occlusion in those lost to follow-up. Our institutional practice is to perform at least 1 posttreatment catheter angiogram, and we are not aware of any symptomatic vessel occlusions during the study period.
Target vessel and aneurysm neck diameter is naturally variable, with a single average number being an oversimplification. Attention to specific parts of the construct, especially leading and trailing edges, may be needed.
The tissue overgrowing the PED is poorly defined and likely includes endothelium with a mix of subendothelial elements that we refer to collectively as neointima. Some amount of mural thrombus is also not possible to exclude. A more tissue-specific in vivo evaluation would require optical coherence tomography.29
2D DSA measurements have limitations in precision. While absolute measurement errors are possible, relative errors in comparative DSA images are unlikely. We did not perform internal correlation to other uninvolved arteries, to control diameter change in all arteries between treatment and follow-up (due to differences in anesthesia for example). Another limitation of 2D DSA is an inability to accurately measure device length. The quantitative evaluation of delayed foreshortening remains unstudied.
Finally, this study did not focus on treatment efficacy. We believe that previously described factors rather than the degree of endothelialization influence occlusion status.30
CONCLUSIONS
The vascular reactive process following PED implantation involves 2 key mechanisms: an endothelialization-related decrease in arterial diameter and outward remodeling (dilation) of the target arterial segment (surmised from the increase in implant diameter with time), mitigating against endothelialization-related narrowing. The extent of outward remodeling is directly influenced by the choice of PED diameter. This influence presents a clinical balancing act: Opting for a larger PED size (judicious oversizing) can enhance remodeling but leads to a reduction in metal coverage. Despite this trade-off, the PED demonstrates consistent effectiveness while preserving functional arterial diameter. Collection of similar data for other devices is essential.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received November 27, 2023.
- Accepted after revision February 6, 2024.
- © 2024 by American Journal of Neuroradiology